
Benefits of lung modeling by high-quality three-dimensional computed tomography for thoracoscopic surgery
Introduction
In recent years, the development of imaging modalities and the widespread use of low-dose helical computed tomography (CT) have contributed to an increase in the detection of early stage lung cancer (1,2). At present, the standard treatment for early stage lung cancer is surgical resection. Recently, video-assisted thoracoscopic surgery (VATS) has become widely used as a less invasive procedure. In fact in the US, of 55,972 patients who underwent lobectomy for non-small-cell lung carcinoma (NSCLC) performed at 905 commission on cancer accredited hospitals over a 3-year study period between 2010 and 2012, 17,072 (30.5%) patients underwent a VATS approach (3). Moreover, the European Society of Thoracic Surgeons database showed that the number of VATS procedures for lung cancer dramatically increased to 18.8% between 2010 and 2012 (4). The Japanese Association for Thoracic Surgery Annual Report for 2016 indicated that VATS for lung cancer was performed in 64.3% of patients (26,188) in 2015, whereas the operation for lung cancer involving all procedures in general thoracic surgery was performed in 50.5% of patients (5). Minimally invasive surgeries, such as VATS lobectomy or segmentectomy, require precise anatomical knowledge of the pulmonary vessels and bronchi for each patient. Thus, preoperative information of the surgical anatomy is anticipated to substantially contribute to the safety of each operation.
The technology of three-dimensional (3D) reconstruction generated from multi-detector computed tomography (MDCT) imaging enables surgeons to recognize the anatomic structures of each patient preoperatively. Furthermore, 3D imaging reflects the actual lung structures or tumors more accurately than two-dimensional (2D) imaging that is used by conventional CT imaging. In this review, we discuss the pragmatic features of the 3D imaging technology for thoracoscopic lung cancer surgery, focusing on complicated surgeries or preoperative evaluation. We also present our perspectives on the 3D imaging technology and surgical resection for lung cancers.
Three-dimensional imaging software technology
The development of MDCT has enabled the rapid scanning of large body areas simultaneously with less artifacts from respiratory motion. Subsequently, this technology has facilitated 3D image reconstruction in general thoracic surgery using different types of software.
In 2003, Watanabe et al. used 3D-CT pulmonary angiography to evaluate the branch patterns of pulmonary arteries (PAs) before anatomic pulmonary resection (6). Of 14 patients with primary lung cancer who underwent anatomic pulmonary resection, 98% (84 of 86) of the PA branches were successfully identified on preoperative 3D-CT images. Fukuhara et al. reported 49 lung cancer patients who were examined by preoperative 3D-CT angiography before complete VATS lobectomy (7). The intraoperative findings showed that 95.2% (139 of 146) of the PA branches were precisely detected on the 3D-CT images. Some of the undetected PA branches in the studies of Watanabe et al. and Fukuhara et al. were reported to be less than 1.5 mm and 2 mm in diameter, respectively.
In 2009, Akiba et al. reported the 3D images of the bronchial trees of 27 lung cancer patients who underwent lobectomy or segmentectomy, as well as the 3D images of the pulmonary arteries and veins. The detected ratio of the pulmonary arteries of the patients on the 3D images was 95% (25 of 27) when compared with the actual resected pulmonary arteries. All of the resected bronchi in the patients were identified on the 3D images (8). There are also several studies that showed the 3D images of the bronchial trees and pulmonary vessels of lung cancer patients who underwent pulmonary resection (9,10).
We previously described the usefulness of 3D-CT lung modeling using a high-speed 3D image analysis system (Synapse Vincent, Fuji Film Co., Ltd., Tokyo, Japan) and reported the ability of recent 3D imaging software to generate the segmented lung parenchyma as well as the bronchial trees and pulmonary vessels (11). The system generates 3D images using digital imaging and communication in medicine (DICOM) data in conventional enhanced CT scanning. This avoids exposure of patients to excessive radiation or to a leaked iodinated contrast medium in their arms. The processing time for visualizing the pulmonary vessels, tracheobronchial trees, and lung parenchyma is approximately 5 minutes. After reconstructing the 3D images, the system allows investigators to simulate each operation, evaluate the targeted tumors, or measure the surgical margins from the tumors.
Applications of 3D imaging for thoracoscopic surgery
Hagiwara et al. previously evaluated the short-term outcome of VATS lobectomy or segmentectomy as well as the detection ratio of the PA branches using 3D imaging analysis (12). They analyzed 179 lung cancer patients who underwent VATS anatomical lung resection. The surgical outcomes were associated with operative complications or operative time. Preoperative 3D imaging tended to reduce the occurrence of complications (risk ratio: 2.852, P=0.074). Moreover, preoperative 3D imaging was significantly associated with total operative time on multivariate analysis (risk ratio: 2.282, P=0.021).
Anomalous pulmonary veins have also been detected by preoperative 3D imaging analysis. In particular, Akiba et al. reported a patient who underwent VATS right lower lobectomy for lung cancer avoiding the resection of an anomalous lateral part of the middle lobe vein (V4) draining into the right inferior pulmonary vein (13). Akiba et al. emphasized that preoperative 3D imaging enabled them to fully comprehend the patient’s vascular anatomy before the operation.
Fukuta et al. described 2 lung cancer patients who had anomalous veins as detected by preoperative 3D imaging and who received VATS lobectomies (14). Sumitomo et al. reported a lung cancer patient who had transposition of the right pulmonary artery and the V1 through V3 segments of the pulmonary vein (15). The anomalous vessels were detected by preoperative 3D imaging analysis, and VATS right upper lobectomy was performed.
Three-dimensional imaging analysis has also been reported to be useful for robot-assisted thoracic surgery. Specifically, Kajiwara et al. demonstrated the use of preoperative 3D imaging analysis not only for intraoperative navigation, including the detection of tumors or surrounding tissues, but also for knowing the best positioning of the robotic arms and instruments preoperatively (16).
Three-dimensional imaging and complex thoracoscopic procedures
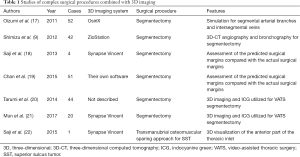
The studies describing complex thoracoscopic procedures combined with 3D imaging are shown in Table 1.
Full table
In 2011, Oizumi et al. reported on complete thoracoscopic segmentectomy in 52 patients while simulating the segmental arterial branches, intersegmental veins that were to be preserved, and venous branches of the affected segment that were to be divided using 3D reconstructing software (OsiriX) (17). Oizumi et al. classified thoracoscopic segmentectomy into 3 categories based on technical difficulty as follows: easy, fairly difficult, and difficult. Using 3D reconstruction to identify the intersegmental veins and then dissecting along them, Oizumi et al. could perform segmentectomy using a complete thoracoscopic approach which can be performed even for anatomically difficult segments.
The benefits of 3D-CT angiography and bronchography for 27 hybrid VATS segmentectomies and 15 complete VATS segmentectomies have also been demonstrated by Shimizu et al. (9). They utilized a workstation with volume-rendering reconstruction software (Ziostation, Tokyo, Japan) to generate 3D-CT angiography images. Additionally, 3D reconstruction of the bronchial tree was performed by mathematical morphology-based 2D segmentation, and further restoration was achieved by the manual addition of segments from the 2D axial images to the 3D image. The distance between the intersegmental veins and the tumor was measured using the 3D-CT image. Shimizu et al. applied 3D-CT angiography and bronchography for segmentectomy to understand the positional relationship between the segmental veins and the bronchi as well as the anatomical variations of the pulmonary veins and bronchi. They described the difficulty of identifying the intrasegmental and intersegmental veins for segmentectomy using only 3D-CT angiography, because the branches of the pulmonary veins were associated with the bronchi in various patterns.
Preoperative virtual segmentectomy using a 3D imaging system (Synapse Vincent) has been shown by Saji et al. to contribute to making a decision regarding the appropriate anatomical segmentectomy and curative resection for thoracic malignancies (18). Their virtual segmentectomy showed a visualized segmental area based on the calculation of bronchial ventilation, the segmental surface resulting in the determination of the resected pulmonary vessels including the intersegmental veins and bronchi, and the distance of the surgical margin. The 3D imaging system could visualize the appropriate intersegmental plane which they simulated, allowing them to decide on their ideal segmentectomy procedure preoperatively with the achievement of a sufficient surgical margin. Sequentially, they assessed the actual surgical margins after segmentectomy and compared them with the preoperative surgical margins generated by their 3D imaging system. The preoperative surgical margins were slightly overestimated, acknowledging this as a limitation of their preliminary study. Saji et al. indicated their resolve to further develop their procedure and reduce the discrepancies between the preoperative surgical margins and the actual surgical margins.
In their pilot study, Chan et al. described their own 3D imaging software which provided automated lung segmentation, tumor localization, and the estimated margins of surgical resection (19). Their study included 51 patients who underwent segmentectomy or lobectomy. Lung segmentation was successful in 73% of the patients using their 3D imaging system. Chan et al. indicated the poorly visualized fissures or poor airway visualization as the reason for the failure of lung segmentation in the other patients. Additionally, the 3D imaging software analyzed the surgical margins for segmentectomy and lobectomy. Chan et al. assessed the predicted surgical margins by comparing them with the actual surgical margins. They found no significant differences in the surgical margins for both segmentectomy (P=0.781) and lobectomy (P=0.681). The positive predictive value was 87% when they predicted a marginal clearance greater than 1 cm; the positive predictive value was 75% when the ratio of the surgical margin to the tumor diameter was predicted to be greater than 1.
Three-dimensional reconstructed imaging and indocyanine green (ICG) have also been used for VATS segmentectomy by Tarumi et al. (20) and Mun et al. (20,21). During the preoperative evaluation, dominant pulmonary arteries for a targeted segmental lung were identified. After the dominant pulmonary arteries and segmental bronchus were divided in the operation, ICG was injected into a peripheral vein. Under infrared light, the appearance of the lung in the areas with blood flow gradually changed, whereas the appearance of the lung in the areas without blood supply remained pale. This method allowed surgeons to recognize the segmental fissures easily for anatomical segmentectomy even with a limited surgical view such as in VATS. Moreover, Tarumi et al. identified the intersegmental lines in 84% of their patients (20). Mun et al. demarcated a border between the targeted segments and preserved lung clarity, and marked the resected line on the lung surface in 95% of their patients (21). Moreover, Mun et al. evaluated the time it took for the demarcation lines to appear after ICG injection and the time the lines lasted, namely, 20 s (10–100 s) and 180 s (90–300 s), respectively.
Preoperative 3D-CT imaging analysis has also been applied to a patient with superior sulcus tumor which invaded the anterior part of the thoracic inlet including the clavicle, sternoclavicular joint, first rib, subclavian vessels, and brachial plexus (22). The patient underwent a transmanubrial osteomuscular-sparing approach and an additional third anterolateral thoracotomy with a hemi-clamshell incision to achieve complete tumor resection.
Prediction of postoperative pulmonary function by 3D imaging
Several studies on the prediction of postoperative pulmonary function by 3D-CT imaging have been conducted (Table 2).

Full table
Ueda et al. compared postoperative ground glass opacity (GGO) (FEV1.0) calculated by 3D-CT imaging analysis with postoperative FEV1.0 measured by the actual pulmonary function test in 30 patients who underwent pulmonary resection (23). They initially demonstrated a high correlation coefficient of predictive postoperative FEV1.0 between the conventional segment-counting method and the 3D-CT imaging analysis (correlation coefficient =0.984, P<0.001). Subsequently, they showed that the measured postoperative FEV1.0 was higher than the predictive postoperative FEV1.0 by 3D-CT imaging analysis with an average difference of 16.9% in patients with ≥10% of the emphysema index. The measured FEV1.0 value was similar to the values analyzed by 3D-CT with an average difference of 2.3% in patients with <10% of the emphysema index (P=0.042). Their results suggested that the predictive postoperative pulmonary function was underestimated in patients with ≥10% of the emphysema index.
Similarly, Kobayashi et al. evaluated the predictive residual pulmonary function by the 3D-CT volumetry method in 53 patients who underwent anatomical pulmonary resection for primary lung cancer, and thereafter compared this pulmonary function with that evaluated by the conventional segment counting method (24). After analyzing their 2 methods, they investigated the differences between the predictive values and the actual values shown in the results of the postoperative pulmonary function tests. Although there was no significant difference between the conventional segment counting method and the 3D-CT imaging analysis, the linear correlation coefficients between the values of predictive postoperative pulmonary function and those of the actual measured pulmonary function were 0.57–0.78 in patients with chronic obstructive pulmonary disease (COPD) based on the Global initiative of Obstructive Pulmonary Disease (GOLD) classification; the correlation coefficients were approximately 0.9 or more in patients without COPD. Furthermore, the correlation coefficients of the 3D-CT volumetry method tended to be higher than those of the segment-counting methods in the patients with COPD. Kobayashi et al. concluded that the 3D-CT volumetry method enabled the prediction of a more accurate residual pulmonary function than the conventional segment counting method, particularly in patients with COPD.
Importantly, there are also some studies on the relationship between 3D-CT imaging analysis and postoperative complications, particularly in patients with emphysema. Kawakami et al. evaluated the association between preoperative 3D-CT volumetry and postoperative complications in 309 patients who underwent lobectomy for primary lung cancer (25). The percentage of low attenuation volume (LAV%) was defined as the proportion of the volume of voxels with attenuation values less than –950 HU of thresholds against the total lung volume. Their cohort included 133 patients with COPD based on the GOLD classification. Their results showed that LAV% was significantly associated with postoperative complications on multivariate analysis (P=0.006), whereas FEV1.0 was not significantly associated with postoperative complications
Makino et al. illustrated the relationship between postoperative respiratory complications and 3D-CT imaging analysis (26). Their analysis included the percentage of low attenuation area (LAA%), which was the same as the above-mentioned LAV%, and the Goddard score. The Goddard score was evaluated from 6 axial images which were provided from 3 specific slices of each patient. The Goddard score and predictive postoperative FEV1.0 were significantly associated with postoperative respiratory complications and gender. The results also demonstrated that the Goddard score and LAA% were strongly correlated with each other (q=0.713, P<0.001).
Prognostic factors of lung cancer as determined by preoperative 3D imaging
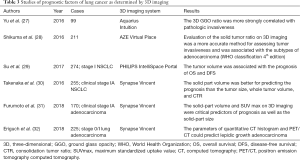
The studies on the prognostic factors of lung cancer as determined by 3D imaging are summarized in Table 3.
Full table
Yu et al. previously assessed the association between tumor invasiveness and parameters generated by 3D-CT imaging analysis (27). They analyzed 99 patients who underwent complete resection for stage IA lung adenocarcinoma. Tumor invasiveness was defined as invasive adenocarcinoma based on the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification, which is based on the World Health Organization (WHO) classification 4th edition. After 3D reconstruction of CT images, 3D solid tumor size and 3D GGO ratio were calculated, as well as one-dimensional (1D) or two-dimensional (2D) size and ratio. The cut-off values were detected by receiver operating characteristic (ROC) analysis, and the sensitivities of the 3D GGO ratio and 3D solid size were 89.7% and 85.3%, respectively; the specificities of the 3D GGO ratio and 3D solid size were 93.5% and 87.1%, respectively. Yu et al. concluded that the 3D GGO ratio was more strongly correlated with pathologic invasiveness than the 3D solid size.
Similarly, Shikuma et al. investigated the relationship between tumor invasiveness and parameters obtained by 3D-CT imaging analysis (28). Their analysis of tumor invasiveness included pathological lymph node metastases, macroscopic pleural invasion, vessel invasion, and lymphatic invasion. The parameters were evaluated by ROC analysis using the 3D solid tumor side and 3D solid tumor ratio, and compared with the 1D/2D solid tumor size and solid tumor ratio. Interestingly, the accuracy of the 3D solid tumor size was equal to that of the 1D solid tumor size on ROC analysis for each tumor invasive factor. However, the accuracy of the 3D solid tumor ratio was higher than those of the 1D and 2D solid tumor ratios on the ROC analysis. Therefore, Shikuma et al. indicated that the 3D evaluation of the solid tumor ratio was a more accurate method for assessing tumor invasiveness, whereas the 1D evaluation was more appropriate for the evaluation of the solid tumor size. Furthermore, Shikuma et al. analyzed the correlations between the 3D solid tumor ratio and the subtypes of adenocarcinoma based on the WHO classification 4th edition. Their results showed significant differences in the 3D solid tumor ratio for adenocarcinoma in situ (AIS)/minimally invasive adenocarcinoma (MIA) versus lepidic adenocarcinoma, lepidic adenocarcinoma versus papillary adenocarcinoma, and lepidic adenocarcinoma versus acinar adenocarcinoma (P=0.011, P<0.0001, P<0.0001, and P<0.0001, respectively).
Three-dimensional tumor size has also been reported as a prognostic factor in patients with surgically resected lung cancer. In particular, Su et al. evaluated the prognostic impact of tumor volume on 274 patients who underwent surgical resection for stage I NSCLC (29). They performed 3D-CT analysis to calculate tumor volume and the greatest tumor diameter. The cut-off values of the tumor volume for disease-free survival (DFS) and overall survival (OS) were determined using their ROC curves. Based on the cut-off values, a larger tumor volume was found to be a significant poor prognostic factor for OS and DFS. Notably, similar results were observed in the tumors whose greatest tumor diameter was less than 3 cm.
Takenaka et al. investigated the solid part volume and whole tumor volume in 255 patients who underwent surgical curative resection for clinical stage IA NSCLC by 3D volumetric analysis (30). They determined each parameter for recurrence using ROC curves, the whole tumor size, consolidation tumor ratio (CTR), the whole tumor volume, and the solid part volume. Their results showed that the whole tumor volume and solid part volume stratified by the above threshold were significantly associated with DFS in the patients (P=0.02 and P<0.01, respectively). Additionally, the solid part volume was better for predicting the prognosis of clinical stage IA NSCLC than the tumor size, whole tumor volume, and CTR on the multivariate analysis.
The combination of 3D-CT imaging with WHO (PET/CT) for lung cancer surgery has also been recently reported. Furumoto et al. in particular showed that the maximum standardized uptake values (SUVmax), solid part size on high-resolution CT (HRCT), and solid part volume on 3D imaging were critical predictors of prognosis and pathological invasiveness in patients with clinical stage IA adenocarcinoma (31). Their 170 patients underwent PET/CT, HRCT, and 3D reconstruction imaging before the complete resection of lung cancer. The ROC curve allowed them to determine the cut-off values of each parameter, and the prognosis of the patients stratified by those values obviously reflected better or worse. The solid part volume was an independent predictor of DFS on multivariate analysis, whereas the volumetric quantification of the solid part and SUVmax were the most powerful predictors.
On the other hand, Eriguchi et al. assessed the parameters of 3D-CT imaging and PET/CT to predict lepidic growth adenocarcinoma, including AIS, MIA, and lepidic-predominant adenocarcinoma (32). Their study included 225 patients with clinical stage 0 or stage I lung adenocarcinoma. When the quantitative CT histograms and PET/CT assessment of the tumors were utilized, the SUVmax, maximum CT attenuation value, and 75th percentile CT attenuation value enabled them to distinguish lepidic growth adenocarcinoma from other tumors.
Conclusions and future perspectives
The development of 3D imaging technologies has led to the preoperative evaluation of lung functions or tumors, as well as to obtaining more precise information of anatomical structures for lung cancer surgery. The unique visualization of anatomical structures generated by 3D imaging software has enabled surgeons to perform safer and more accurate operations despite performing minimally invasive surgery with a limited field of vision. Moreover, 3D imaging requires no additional examination for patients, and the process of reconstructing 3D images is simple and takes only a few minutes. Additionally, the visualization technology can predict the risk of postoperative complications while it analyzes factors associated with lung functions. Moreover, 3D imaging can predict histopathological features (e.g., lepidic growth pattern) or prognostic factors with/without the combination of PET/CT examination.
Importantly, the preoperative data and feedback from the 3D imaging may be used for developing different types of real-time navigation software, such as a global positioning system. This will enable surgeons or robots to more accurately recognize anatomical structures and perform operation easier. On the other hand, considerable efforts must be exerted to identify parameters for the prediction of histopathological features, tumor invasiveness, or prognostic factors by 3D imaging.
In the future, we anticipate that the 3D imaging technology will enable the prediction of the molecular features of lung cancer in combination with other examination devices without the need for pathological examination.
Acknowledgments
We thank Dr. Edward Barroga (
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “New Technologies for Advanced VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.01.02). The series “New Technologies for Advanced VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research T. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Abdelsattar ZM, Allen MS, Shen KR, et al. Variation in Hospital Adoption Rates of Video-Assisted Thoracoscopic Lobectomy for Lung Cancer and the Effect on Outcomes. Ann Thorac Surg 2017;103:454-60. [Crossref] [PubMed]
- Begum S, Hansen HJ, Papagiannopoulos K. VATS anatomic lung resections-the European experience. J Thorac Dis 2014;6:S203-10. [PubMed]
- Committee for Scientific Affairs TJAfTS. Thoracic and cardiovascular surgery in Japan during 2015: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2018;66:581-615. [Crossref] [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92; discussion 392. [Crossref] [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Eguchi T, Takasuna K, Kitazawa A, et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur J Cardiothorac Surg 2012;41:893-7. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgerydagger. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Anomalous pulmonary vein detected using three-dimensional computed tomography in a patient with lung cancer undergoing thoracoscopic lobectomy. Gen Thorac Cardiovasc Surg 2008;56:413-6. [Crossref] [PubMed]
- Fukuta K, Shimada Y, Hagiwara M, et al. High-quality 3-dimensional imaging for patients with anomalous pulmonary veins. Asian Cardiovasc Thorac Ann 2015;23:585-7. [Crossref] [PubMed]
- Sumitomo R, Fukui T, Otake Y, et al. Video-assisted thoracoscopic lobectomy with an anomalous pulmonary vein. J Thorac Cardiovasc Surg 2016;152:1398-9. [Crossref] [PubMed]
- Kajiwara N, Akata S, Hagiwara M, et al. High-speed 3-dimensional imaging in robot-assisted thoracic surgical procedures. Ann Thorac Surg 2014;97:2182-4. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Saji H, Inoue T, Kato Y, et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 2013;17:227-32. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Saji H, Kato Y, Shimada Y, et al. Three-dimensional multidetector computed tomography may aid preoperative planning of the transmanubrial osteomuscular-sparing approach to completely resect superior sulcus tumor. Gen Thorac Cardiovasc Surg 2015;63:627-31. [Crossref] [PubMed]
- Ueda K, Tanaka T, Li TS, et al. Quantitative computed tomography for the prediction of pulmonary function after lung cancer surgery: a simple method using simulation software. Eur J Cardiothorac Surg 2009;35:414-8. [Crossref] [PubMed]
- Kobayashi K, Saeki Y, Kitazawa S, et al. Three-dimensional computed tomographic volumetry precisely predicts the postoperative pulmonary function. Surg Today 2017;47:1303-11. [Crossref] [PubMed]
- Kawakami K, Iwano S, Hashimoto N, et al. Evaluation of emphysema using three-dimensional computed tomography: association with postoperative complications in lung cancer patients. Nagoya J Med Sci 2015;77:113-22. [PubMed]
- Makino Y, Shimada Y, Hagiwara M, et al. Assessment of emphysema severity as measured on three-dimensional computed tomography images for predicting respiratory complications after lung surgery. Eur J Cardiothorac Surg 2018;54:671-6. [Crossref] [PubMed]
- Yu WS, Hong SR, Lee JG, et al. Three-Dimensional Ground Glass Opacity Ratio in CT Images Can Predict Tumor Invasiveness of Stage IA Lung Cancer. Yonsei Med J 2016;57:1131-8. [Crossref] [PubMed]
- Shikuma K, Menju T, Chen F, et al. Is volumetric 3-dimensional computed tomography useful to predict histological tumour invasiveness? Analysis of 211 lesions of cT1N0M0 lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;22:831-8. [Crossref] [PubMed]
- Su XD, Xie HJ, Liu QW, et al. The prognostic impact of tumor volume on stage I non-small cell lung cancer. Lung Cancer 2017;104:91-7. [Crossref] [PubMed]
- Takenaka T, Yamazaki K, Miura N, et al. The Prognostic Impact of Tumor Volume in Patients with Clinical Stage IA Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1074-80. [Crossref] [PubMed]
- Furumoto H, Shimada Y, Imai K, et al. Prognostic impact of the integration of volumetric quantification of the solid part of the tumor on 3DCT and FDG-PET imaging in clinical stage IA adenocarcinoma of the lung. Lung Cancer 2018;121:91-6. [Crossref] [PubMed]
- Eriguchi D, Shimada Y, Imai K, et al. Predictive accuracy of lepidic growth subtypes in early-stage adenocarcinoma of the lung by quantitative CT histogram and FDG-PET. Lung Cancer 2018;125:14-21. [Crossref] [PubMed]
Cite this article as: Kudo Y, Ikeda N. Benefits of lung modeling by high-quality three-dimensional computed tomography for thoracoscopic surgery. Video-assist Thorac Surg 2019;4:4.


