
Cost reduction in video-assisted thoracoscopic lobectomy
VATSL: history and current usage
Thoracoscopy was first introduced to thoracic surgery in 1909, but it was not until the late 1980s and early 1990s that video-assisted thoracoscopic surgery (VATS) began in earnest (1). When the first large case study was published in 1993, VATS lobectomy (VATSL) was far from routine (2). Of the 1,820 patients who underwent a VATS procedure, 439 (24.1%) were eventually converted to thoracotomy, and only 38 (2.1%) underwent VATSL. Additionally, VATSL was associated with higher rates of prolonged air leak and longer operating room times compared to thoracotomy lobectomy (THORL). The authors concluded that: “video-assisted lobectomy remains experimental with the potential for major complications.”
Adoption of VATSL over THORL has advanced gradually over the 1990s and 2000s with the publication of a series of papers demonstrating shorter length of stay (3-9) and slightly lower complication rates (3-5,8,10-13). Further studies showed similar, if not better, oncologic (6,14-17) and quality of life outcomes between the two procedures (18,19). Most thoracic surgeons have been convinced that VATS lobectomy is the ideal approach to lobectomy for carefully staged, clinical stage I patients, and many believe it is appropriate for patients with N1 and even N2 disease as well. Centers across the United States and the world have now become more familiar with these techniques (20). Today, guidelines from organizations including the American College of Chest Physicians recommend VATSL over THORL for clinical stage I non-small cell lung cancer (21). Submissions to this journal have called for VATSL to be declared the standard of care for early stage lung cancer (22).
VATSL overall and in-hospital costs
With the rise of VATSL and increasing evidence of the clinical benefits of VATSL over THORL, further attention has been paid to the economic implications of this transition. The increasing number of lung cancer patients who are covered by insurance systems which provide a fixed, global payment for an episode of care, regardless of the specific costs incurred during that episode, have made health-care providers particularly interested in the costs of pulmonary lobectomy. This is of particular interest as this is one of the most common in-patient thoracic operations performed. Given the established shorter length of stay for VATSL vs. THORL patients, at first glance it would appear reasonable to expect that overall hospital costs would also be decreased with adoption of VATSL.
This correlation was indeed demonstrated on a large scale by Swanson and colleagues in 2012 (23). In a retrospective cohort of 3,961 patients, compared to THORL, VATSL was associated with lower rates of adverse events (P=0.019), shorter length of stay (7.83 vs. 6.15 days, P<0.001), and lower hospital costs ($21,016 vs. $20,316, P=0.027). The authors conclude that their study demonstrates “strong evidence showing that VATS lobectomy for lung cancer has both clinical and economic advantages over traditional open thoracotomy for lobectomy.”
Other studies have investigated whether VATSL is associated with lower costs after discharge. In 2014, Farjah and colleagues queried a database of 9,962 patients, finding that VATSL 90-day costs were lower than those of THORL by $3,476 (24). However, they determined that the primary driver of these decreased VATSL costs compared to THORL was the reduced rate of prolonged length of stay (greater than 14 days) after surgery, rather than the smaller difference observed in re-admission rates or emergency department utilization after discharge. Like Swanson, Farjah found hospitalization costs to be significantly lower in VATSL than in THORL.
While these large retrospective database studies provide evidence for decreased in-hospital costs of VATSL compared to THORL, other studies have found no significant difference in hospitalization cost (6,25-27). In a particularly large dataset of 13,619 patients, Gopaldas and colleagues found no statistically significant difference in hospitalization costs for VATSL compared to THORL (25). Similarly, in their 2009 retrospective database study of 12,958 patients, Farjah and colleagues found decreased length of stay associated with VATSL, but no cost benefit (6).
Several authors have provided evidence to explain these contradictory data. In his 2016 review of VATSL costs, Brunelli discusses the population differences in various VATSL costs studies that may explain the discordance (28). It was noted early on that the surgeon may also play a part in explaining this phenomenon. Swanson and colleagues in their 2012 database study found that surgical experience and volume significantly impacted costs (23). For VATSL, hospitalization costs for low-volume surgeons (less than 16 surgeries in a 6-month period) were nearly $4,000 higher than those for high-volume surgeons.
VATSL intraoperative costs—the impact of intraoperative device use
Other authors have focused on the higher intraoperative costs for VATSL vs. THORL, which has been proposed to neutralize or outweigh the cost savings that would derive from shorter length of stay with VATSL compared to THORL (23,24,27,29-31). Studies from 1993 to present have demonstrated longer operating room times for VATSL compared to THORL, with intraoperative costs making up a large portion of total VATSL hospitalization costs. For example, Deen and colleagues found that intraoperative costs were the largest category of expenses for VATSL (32). Likewise, Nakajima and colleagues found that intraoperative costs accounted for 63% of hospitalization costs for their entire cohort (29).
If thoracic surgeons, then, wish to reduce VATSL costs, the operating room is a good first place to begin. Casali and Walker, in their 2009 study, found VATSL to be less expensive overall compared to THORL (31). They found that VATSL operating room costs were nearly twice as high as those of THORL, but that this was offset by the significantly reduced hospitalization length compared to THORL. Of note, they found that intraoperative VATSL costs varied significantly by lobe and type of resection. They attribute this difference mainly to the different needs for disposable instruments such as stapler reloads. They cautioned that their findings may not be generalizable, as hospital policies and local taxes may influence the degree to which these disposables influence overall hospitalization costs.
Similar conclusions have been found in studies across the world. In the United States, Khullar and colleagues showed that intraoperative costs, and specifically stapler utilization, were a primary factor in overall hospitalization cost for VATSL at their institution (33). Importantly, they also noted that of all VATSL hospitalization costs, intraoperative and stapler costs had some of the greatest variability, and as such could be a prime target for cost reduction strategies. In Korea, Cho and colleagues found that only surgical materials were significantly more costly for VATSL than THORL. In this study as well, these material costs varied significantly based on type of resection performed (34). These studies clearly demonstrated that intraoperative materials and devices are a significant contributor to overall VATSL hospitalization cost.
Cost reduction strategies that thoracic surgeons can undertake
One method to control VATSL (and also THORL) costs is to attempt to reduce post-operative length of stay. In their previously discussed paper, Khullar and colleagues determined that while intraoperative costs contributed heavily to total VATSL hospitalization costs, length of stay also made up a significant portion (33). They recommend implementing standardized protocols to optimize ancillary service coordination to allow patients to return home sooner, thus reducing total costs. Other authors, too, have described clinical pathways specifically designed for thoracic surgery patients to have a more standardized and seamless transition to discharge (35-38). Pathways such as these have been investigated by groups with an eye towards their effect on overall cost (39-42). One study by Schwarzbach and colleagues directly compared VATS patients enrolled in a clinical pathway to those who were not. They found that this intervention reduced cost by 1,510 Euros per stay, with that improvement most attributable to decreased length of stay (39). Zehr and colleagues also found decreased length of stay with clinical care pathway implementation, and Wright and colleagues found a mean cost reduction of $1,271 per patient (40,41).
While reducing post-operative length of stay by standardizing perioperative care via care pathways is an important factor in reducing VATSL hospitalization costs, surgeons are also able to personally reduce the significant cost contributed during the operation itself. As discussed in the previous section, intraoperative costs, and specifically intraoperative devices, constitute a significant portion of VATSL total hospitalization cost.
Our group recently published a study looking at costs of VATSL and THORL and the effect of intraoperative disposable instrument/device utilization on total cost (43). We found, comparing the costs incurred by two surgeons, that the increased costs of VATS lobectomy by one surgeon vs. those by a more cost-conscious surgeon resulted almost entirely from increased intraoperative costs (Figure 1). Further, the operating room cost of VATSL compared to THORL is also largely attributable to this surgeon-specific intraoperative device utilization (Figure 2). Within our institution, a cost-conscious surgeon who made it a policy to avoid expensive, disposable instruments had VATSL total hospitalization costs approximately 30% lower than THORL costs, while the less cost-conscious surgeon’s VATSL and THORL overall costs did not significantly differ. The overall hospital costs per case of VATSL for the more expensive surgeon were 24% higher than those for the less expensive surgeon. While the cost-conscious surgeon’s stapler costs were lower than the other surgeon’s, the difference in cost between the surgeons due to all other disposables (e.g., surgical sealants, energy devices, disposable ports) was more important than the effect of less staple load use. The cost-savings achieved intraoperatively during VATSL by the cost-conscious surgeon did not result in any difference in outcomes between the two surgeons.
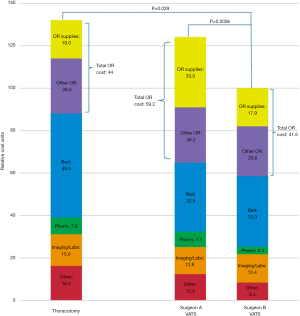
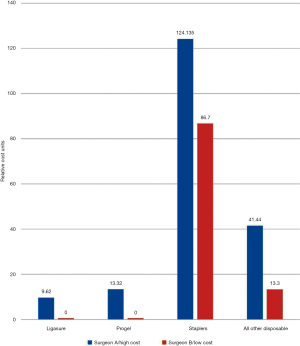
On the basis of these results, we argue that thoracic surgeons can and should make a conscious decision to only very selectively use expensive, disposable equipment. We should not utilize expensive equipment when there is a reasonable, less expensive option which provides equal results with a similar duration of operation. We have, since publishing that paper, been working to apply a project in our institution’s operating rooms to have the cost of each device placed on a label on the wrapping materials containing that device. Our plan is twofold: (I) all surgeons will go over their operating room procedure sets and selectively remove any devices that they believe they may not require; (II) during operations, when a surgeon asks for a disposable device to be opened, the circulating nurse will be instructed to read the cost of that device out loud to the surgeon—only then can the surgeon make the final decision that he or she would like the device to be opened and used.
Other authors have also recently emphasized surgeon ability to reduce intraoperative costs. In a 2018 editorial in the Journal of Thoracic and Cardiovascular Surgery in response to our article, D’Amico concludes that comparison of surgeon costs “is critical if we are soon to make judgments regarding the cost-effectiveness” of various thoracic surgical techniques (44). Demmy, in agreement, suggests that “expensive, disposable items such as wound protectors, energy devices, and so on are not needed in every case. Staff should open these on demand only. Surgeons sharing hospital resources should discuss standardizing their setups, and hospital systems can facilitate these conversations by providing comparisons between providers and reporting costs immediately at the end of each procedure” (45).
While we feel strongly that intraoperative device use can be judiciously reduced without increasing complications or quality of surgery, we do of course caution surgeons not to reduce expenses at the risk of undermining patient safety. Of course, utilizing fewer stapler reloads may result in lower intraoperative costs, but this is unlikely to reduce overall costs if it leads to more cases of prolonged air leak. And it is hardly worth the savings if patient outcomes are jeopardized. Furthermore, while intraoperative costs are a major contributor to total VATSL hospitalization cost, length of stay and other postoperative care costs are of course also influenced by surgical quality. Many papers point to the extreme variability of postoperative costs, which are influenced heavily by longer hospitalizations, unplanned admissions to the ICU, blood transfusions, and other issues that may be caused by surgical complications whose avoidance must be our primary endeavor (24,33,46,47).
Surgeons who have learned how to do VATS procedures using certain expensive instruments would need to gradually learn how to perform the procedures slightly differently, using less costly, alternative instruments—for example using a hook-cautery for mediastinal lymph node dissection instead of a more expensive energy device. This shift cannot be done precipitously. Other shifts—for example from disposable to reusable ports—will be much easier to adopt. Lastly, we do not mean to imply that all newer (and thus likely expensive) intraoperative devices should be abandoned—indeed, innovation in surgery often requires large initial financial investment. Our emphasis is to point out that surgeons should consider cost-effectiveness when determining which supplies to utilize, as there is little doubt that the same operation can be done at substantially less cost, and with the same outcome, if one expends just a little bit of energy towards cost-consciousness.
Conclusions and next steps
In an atmosphere of increasing healthcare cost scrutiny, determining the primary factors leading to hospital costs associated with a surgical procedure are of the utmost importance. For thoracic surgeons, this includes determining both the cost-effectiveness of VATSL and discussing methods to safely reduce the overall costs of the procedure.
Methods proposed to reduce costs associated with VATSL include streamlining patient care pathways and discharge processes, as unnecessary hospital days clearly add to total hospitalization costs. The most effective cost-saving role for surgeons, however, will likely be to focus on the immediate impact we can make in our operating rooms. By emphasizing hospital and system-wide efforts to enable intraoperative cost-consciousness with regard to disposable and non-essential surgical adjuncts, we can substantially reduce costs of VATSL. These include such actions as increased availability of information about the costs associated with each device to surgeons. At our institution, we have begun a process to make all surgeons aware of the cost of each disposable instrument before they commit to opening and using it, so that they have the autonomy to determine if the higher costs that would be incurred are necessary for a particular patient’s case.
While the results of comparative analyses of total hospitalization costs for VATSL compared to THORL have been inconsistent, this uncertainty is largely due to the potentially high costs of intraoperative devices and adjuncts that many surgeons use to perform VATSL. With careful surgical instrumentation selection, VATSL costs can be reduced to levels well below those of THORL. With shorter length of stay for VATSL compared to THORL as well as equivalent or improved additional clinical outcomes, there is little doubt that it would be a consistently more cost-effective procedure than THORL if we could keep the intraoperative costs within reason. These cost-reduction steps will of course also become important as we have moved into the era of comparing the costs of VATSL to robotic lobectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “New Technologies for Advanced VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.12.06). The series “New Technologies for Advanced VATS” was commissioned by the editorial office without any funding or sponsorship. JBS serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jul 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobaeus HC. Ueber die Moglichkeit die Zystoskopie bei Untersuchung seroser Honlungen anzuwenden. Munchen Med Wchenschr 1910;57:2090-2.
- Hazelrigg SR, Nunchuck SK, LoCicero J. Video Assisted Thoracic Surgery Study Group data. Ann Thorac Surg 1993;56:1039-43; discussion 1043-4. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Farjah F, Wood DE, Mulligan MS, et al. Safety and efficacy of video-assisted versus conventional lung resection for lung cancer. J Thorac Cardiovasc Surg 2009;137:1415-21. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, discussion 28-9.e1.
- Watson TJ, Qiu J. The Impact of Thoracoscopic Surgery on Payment and Health Care Utilization After Lung Resection. Ann Thorac Surg 2016;101:1271-9; discussion 1979-80.
- Berry M, Villamizar-Ortiz N, Tong B, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 1051-2. [Crossref] [PubMed]
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Flores RM, Ihekweazu UN, Rizk N, et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2011;141:59-64. [Crossref] [PubMed]
- Burfeind WR Jr, Jaik NP, Villamizar N, et al. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2010;37:827-32. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Seder CW, Raymond D, Wright CD, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:1304-7. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Milman S, Ng T. Has the time come to declare video-assisted thoracic surgery lobectomy the standard of care for early stage lung cancer? Video-assist Thorac Surg 2017;2:66. [Crossref]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Farjah F, Backhus LM, Varghese TK, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg 2014;98:191-6. [Crossref] [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. [Crossref] [PubMed]
- Piwkowski C, Gabryel P, Galecki B, et al. High costs as a slow down factor of thoracoscopic lobectomy development in Poland - an institutional experience. Wideochir Inne Tech Maloinwazyjne 2013;8:334-41. [Crossref] [PubMed]
- Rodgers-Fischl PM, Martin JT, Saha SP. Video-Assisted Thoracoscopic versus Open Lobectomy: Costs and Outcomes. South Med J 2017;110:229-33. [Crossref] [PubMed]
- Brunelli A. Cost analysis of VATS approaches. Video-assist Thorac Surg 2016;1:26. [Crossref]
- Nakajima J, Takamoto S, Kohno T, et al. Costs of videothoracoscopic surgery versus open resection for patients with of lung carcinoma. Cancer 2000;89:2497-501. [Crossref] [PubMed]
- Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [Crossref] [PubMed]
- Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg 2009;35:423-8. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Khullar OV, Fernandez FG, Perez S, et al. Time is Money: Hospital Costs Associated With Video-Assisted Thoracoscopic Surgery Lobectomies. Ann Thorac Surg 2016;102:940-7. [Crossref] [PubMed]
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [Crossref] [PubMed]
- Ardò NP, Loizzi D, Panariti S, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS group: nursing care program. J Thorac Dis 2018;10:S529-34. [Crossref] [PubMed]
- Sihoe AD. Clinical pathway for video-assisted thoracic surgery: the Hong Kong story. J Thorac Dis 2016;8:S12-22. [PubMed]
- Das-Neves-Pereira JC, Bagan P, Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer lobectomy: a five-year experience. Eur J Cardiothorac Surg 2009;36:383-91; discussion 391-2. [Crossref] [PubMed]
- Jones NL, Edmonds L, Ghosh S, et al. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia 2013;68:179-89. [Crossref] [PubMed]
- Schwarzbach MH, Ronellenfitsch U, Wang Q, et al. Effects of a clinical pathway for video-assisted thoracoscopic surgery (VATS) on quality and cost of care. Langenbecks Arch Surg 2010;395:333-40. [Crossref] [PubMed]
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 1998;66:914-9. [Crossref] [PubMed]
- Wright CD, Wain JC, Grillo HC, et al. Pulmonary lobectomy patient care pathway: a model to control cost and maintain quality. Ann Thorac Surg 1997;64:299-302. [Crossref] [PubMed]
- Maruyama R, Miyake T, Kojo M, et al. Establishment of a clinical pathway as an effective tool to reduce hospitalization and charges after video-assisted thoracoscopic pulmonary resection. Jpn J Thorac Cardiovasc Surg 2006;54:387-90. [Crossref] [PubMed]
- Richardson MT, Backhus LM, Berry MF, et al. Intraoperative costs of video-assisted thoracoscopic lobectomy can be dramatically reduced without compromising outcomes. J Thorac Cardiovasc Surg 2018;155:1267-77.e1. [Crossref] [PubMed]
- D'Amico TA. Undoing the gaps in quality, cost, and value. J Thorac Cardiovasc Surg 2018;155:1211. [Crossref] [PubMed]
- Demmy TL. Finally, new conversations about video-assisted thoracoscopic surgical lobectomy. J Thorac Cardiovasc Surg 2018;155:1278-9. [Crossref] [PubMed]
- Medbery RL, Perez SD, Force SD, et al. Video-assisted thoracic surgery lobectomy cost variability: implications for a bundled payment era. Ann Thorac Surg 2014;97:1686-92; discussion 1692-3.
- Brunelli A, Tentzeris V, Sandri A, et al. A risk-adjusted financial model to estimate the cost of a video-assisted thoracoscopic surgery lobectomy programme. Eur J Cardiothorac Surg 2016;49:1492-6. [Crossref] [PubMed]
Cite this article as: Richardson MT, Shrager JB. Cost reduction in video-assisted thoracoscopic lobectomy. Video-assist Thorac Surg 2019;4:1.


