VATS for advanced T status (large tumors, mediastinal invasion and vascular control)
Introduction
In the early 1990s following the rapid adoption of laparoscopic cholecystectomy by general surgeons, thoracic surgeons incorporated minimally invasive procedures for technically straightforward procedures of the chest like pleural biopsy or peripheral lung nodule excision. Despite adoption of minimally invasive lobectomy by a few specialized centers at about the same time, the tipping point for widespread acceptance has only happened recently. This is because of challenges based on availability of adequate technology and surgeon training but also because of concerns of oncologic equivalence (1).
Once programs adopted minimally invasive lung resections as the preferred practice for early stage lung cancer, the quality of the operations improved with better lymph node harvest and expansion of the indications toward higher stages occurred (2). While randomized studies have not been performed, early indications suggest that applying video-assisted thoracoscopic surgery (VATS) or other minimally invasive techniques for more complex resections is safe (1).
In this article, we will briefly review the reports of these early experiences emphasizing the challenges associated with tumors of high T stage. Specific instances of advanced T status such as chest wall invasion, and location requiring pneumonectomy will be addressed by other articles in this issue. Arguably, the technical challenges dealing with tumors that are very large or have invaded structures that are difficult to resect are the main concerns preventing programs from achieving near 100% reliability with minimally invasive lobectomy. Accordingly we will also review the innovations some investigators used to confront these challenges.
Literature review
The criteria that investigators have used to determine whether a tumor’s T status classified the case as an advanced minimally invasive resection are somewhat different between institutions. VATS lobectomy was defined originally as an operation for patients with peripheral malignancy (3). Accordingly, central lung cancers were probably underrepresented in large databases early on and may have contributed to the findings of reduce lymph node yields (4,5).
In 2013, investigators at Duke published their results of tumors that could not be removed by wedge resection to define centrality (6). They demonstrated an increased proportion of such patients over time with more having advanced nodal stations as well. Five hundred and five patients met their criteria for complexity by having tumors greater than 3 cm, centrality, and clinical N1-N3 disease. Interestingly, 23 patients had tumors resected that were over 7 cm.
Pischik and colleagues, in 2014, used a cut point of greater than 5 cm of tumor size to define a locally extensive VATS case as well as extension to the chest wall or mediastinum and previous chemotherapy or radiation treatments (7). Centrality was defined as extension to the lobar bronchus and patients with N1-2 disease were included as well. They also included some criteria that certainly make surgery difficult but were not used by others to designate advanced tumors such as dense pleural adhesions, severe emphysema, and previous surgery.
Some patients with advanced T are included in series describing VATS resection following induction therapy. Many times the T status was not reported such as the report from Huang and colleagues who operated on 43 post-induction patients with Stage IIA to IIIB disease, 9 of whom required sleeve resection (8). Nakanishi used the preoperative stage 2 or greater to defined advanced VATS without specific T-stages but 37% of cases having tumors requiring resections of adjacent structures like vascular or airways outside the usual lobectomy anatomy (9).
More recently, two propensity matched concurrent cohort studies of post induction patients from Duke and Cornell (VATS case N=69 and 40 respectively), showed a trend toward better survival in those that had minimally invasive approaches as well as the usual short-term benefits of less invasive surgery like briefer hospital stays (10,11). In these series, the number of clinical T3 or T4 patients were quite different with 11 in the former and only 1 in the latter. Many more clinical T3-4 patients (N=31) were reported by Chen and colleagues in a series of Stage II-IIIA patients where long term survival was similar to propensity match thoracotomy patients (12).
This research was expanded by multicenter registry in Italy using tumor size >5 cm (cT2b), cT3, cT4 and/or induction chemotherapy as the search criteria to find 454 locally advanced VATS patients (13). These patients were compared to 3266 VATS patients without such criteria and they had, as expected, longer operative times and more conversions 13.0% vs. 9.3%. More importantly, these advanced VATS cases had similar morbidity and mortality rates even when considering the conversions separately.
Other minimally invasive approaches for locally advanced tumors have been reported. A roughly equal mix of Robotic and VATS for post-induction Stage II-IIIA patients had shorter hospitalization and equivalent medium-term survival (14). Uniportal approaches for advanced cases as defined by Roswell Park criteria (see next section) had similar results with better tolerance of adjuvant therapy for the minimally invasive approach (15).
Roswell park experience
In 2011 we published our experience with advanced tumors and used the criteria of T >4 cm, T3-4 (excluding chest wall), or any tumors that required neoadjuvant therapy which based on our institution’s preference was largely chemotherapy (16). Of 95 patients who fulfilled the criteria and were attempted by VATS, 73 were successful with a 25% conversion compared to 19 planned open cases. With an intent-to-treat analysis, there was no evident difference in overall or disease-free survivals.
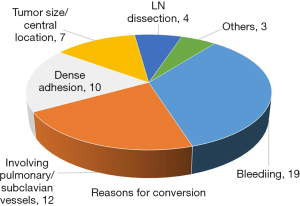
In 2012, we conducted an unpublished update of our series to a total of 224 completed VATS cases and were able to offer a successful minimally invasive resection to 82% of all our patients who achieved these criteria that year. Reasons for conversion for the 55 patients in the updated series are shown in Figure 1.
Like VATS for early stage cancer, we demonstrated shorter hospital stays 6.6±9.4 days for these complex patients who had a successful VATS compared to Conversions (9.1±9.5) or planned open (10.7±9.5, P<0.05) cases. This appeared to be from reductions in complications like pneumonias that were significantly more common in converted or open patients. Preoperative comorbidities, clinical stages, and pulmonary function testing results were similar amongst the patients who underwent a VATS approach compared to those approached by planned thoracotomy. For patients who were attempted by VATS (including conversions) 45.7% went on to receive adjuvant chemotherapy, compared to only 25% of those who underwent planned thoracotomy.
Technical maneuvers
3D videoscope technology
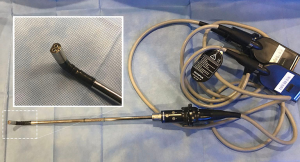
Approaching larger tumors, which are often associated with locally advanced nodal disease, presents specific challenges for visualizing the dissection field, achieving adequate retraction of the tumor itself, as well as extracting the large specimen. Dissection of the hilar and fissure structures may be hindered by tumor bulk. Advances in surgical videoscope technology have helped to overcome certain challenges associated with viewing. Hi-definition flexible tip videoscopes aid in overcoming difficult viewing angles presented by bulky tumors by being able to work around the horizon created by a large tumor. At our institution, our preferred videoscope technology has primarily consisted of flexible tip, hi-definition videoscopes, most recently utilizing 3-D viewing capabilities (Figure 2, ENDOEYE FLEX 3D; Olympus, Center Valley, PA). Smaller diameter 5 mm videoscopes allow for instrumentation to be introduced adjacent to the videoscope offering more possibilities for viewing and retracting. Other quality systems exist (Karl Storz, Germany) and have been studied (17,18). Our anecdotal experience utilizing the Olympus 3D system has been that 3D viewing capabilities aid in the dissection of hilar structures, lymph node dissection, and may lead to a reduction in operating room time. Reports examining the potential effects of 3D viewing compared to 2D viewing exist for laparoscopic and thoracoscopic procedures, with several focusing on thoracic surgery procedures. A large meta-analysis examining 3D vs. 2D for thoracoscopic procedures, largely involving institutions utilizing Karl Storz systems, reported decreased length of hospital stay, shorter operating times, and decreased intraoperative blood loss. No difference was noted in number of lymph nodes dissected with 3D vs. 2D systems (19).
Hemostasis options
When operating on larger tumors with more challenging dissections, management of intraoperative blood loss can be an issue. Various instruments and hemostatic agents can aid in minimizing blood loss associated with large tumor resection. For diffuse oozing from broad surface areas that result from pleural dissections, or parenchymal bleeding, bipolar tissue coagulation with radiofrequency transcollation technology (Aquamantys, Medtronic Minneapolis, MN, United States) has proven to be a very useful tool for minimizing blood loss associated with diffuse oozing. Transcollation technology delivers radiofrequency energy simultaneously with saline for hemostatic sealing and coagulation of soft tissue at the surgical site. Temperatures stay at or below 100 °C, so there is no smoke or char formation when placed in contact with tissue (20,21).
We routinely utilize absorbable hemostatic agents in helping minimize blood loss during complex VATS resections. For small, low volume bleeding, cellulose based agents in the form of sponges, cloth, or powders are very effective at temporizing slow, low pressure bleeding. For larger more significant hemorrhage, such as that from a pulmonary artery, fibrin sealant patches such as Evarrest (Ethicon, Cincinnati, OH, United States) or TachoSil (Baxter, Deerfield, IL, United States) can be directly applied to control bleeding while alternative plans are made for proceeding (conversion, resuscitation, achieving proximal control of the main pulmonary artery at the hilum, etc.).
Trouble shooting tips
A useful trouble shooting checklist that can apply to any VATS case involves the following seven “F’s”.
- Free all adhesions, all lobes.
- Find Somewhere else to work or view the target.
- Pleurae and lymphatic tissue.
- Divide more distally.
- Fissure division (also opens camera angles).
- Partially (outside in).
- Completely (blunt clamp technique).
- Flip order of anatomical divisions (e.g., change to fissure last technique).
- Fill the port/access incisions (“fers”) with extra instrument to aid exposure.
- Traction—counter-traction to define anatomy (e.g., “Two-fer” and vessel loops).
- Fresh planes (e.g., open pericardium).
- Flatten the diaphragm.
Large specimen management
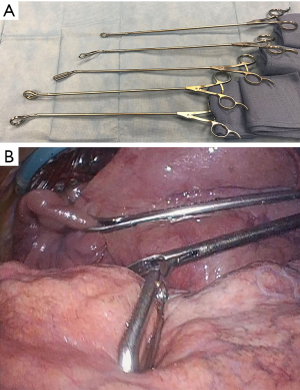
Difficulties in retracting large tumors during thoracoscopic dissection can be overcome effectively utilizing multiple low profile VATS instruments retracting tissue or specimen from multiple angles. As many as five low profile graspers/retractors can be placed through a single thoracoscopic incision to allow retraction from multiple angles and grasping points on the specimen. Our experience has relied heavily on 5 mm thoracoscopic instruments (Figure 3, Sontec Instruments, CO, United States), but multiple producers exist for low profile thoracoscopic surgical instruments. Non-thoracic instruments can be very helpful in providing retraction during thoracoscopy. A laparoscopic flexible liver retractor (Figure 4, Diamond-Flex™, McKesson, Jacksonville, FL) can be used to effectively retract a large tumor by tightening the retractor around the base of the tumor applying leverage. This retractor is also very effective for retracting the diaphragm downwards, and when anchored by a videoscope holder attached to the operating table side bar, can provide additional freedom for working.
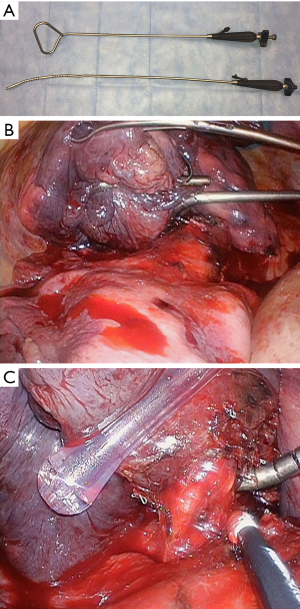
If tumor bulk is preventing safe dissection of hilar or fissure structures, separating tumor from the hilum utilizing a deep wedge resection can facilitate much easier dissection of the remaining structures for the lung resection. Extracting large tumors through small VATS incisions can be problematic. Typically specimen extraction is performed through a 4 cm access incision which is created in the fourth intercostal space. Generous division of the intercostal musculature and the parietal pleura allows for increased flex of the intercostal space. Positioning the incision more anteriorly where the intercostal space widens is also helpful. Another option involves extending the anterior/inferior VATS incision to create an extraction site where ribs can flex the most.
Large extraction sacs made from tear resistant material such as the Anchor tissue retrieval bag (Figure 5, Con-med, Utica, NY, USA) allow for easier placement of tumor into the sac, as well as pulling the specimen through incisions. The largest tumor resections often require an 8×10” sac (Cook Medical, Bloomington, IN, USA). A common pitfall when extracting large specimens is the potential rib fracture that can occur despite all measures taken to prevent it. In anticipation of this, it is reasonable to divide a rib in a controlled fashion (notching the rib) to prevent fracture. If rib fracture does occur, stabilizing the fracture with absorbable rib plates such as the BioBridge (Acute Innovations, Hillsboro, OR, United States) is very helpful in minimizing postoperative pain and improving respiratory mechanics.
Options for removing large tumors without having to spread or divide the ribs in any way have been reported and include non-traditional incision sites such as a midline subxiphoid incision. Kato et al. reported on five patients with T2 and T3 tumors who underwent complete VATS resection with tumor extraction performed through a retro-sternal, extraperitoneal subxiphoid upper abdominal midline incision (22). Postoperative pain management was reported as excellent, presumably due to minimized rib trauma or intercostal nerve irritation.
Vascular management
Large, central tumors create complexity for pulmonary vasculature control by limiting the available vascular length for division and crowding dissection spaces. This effect is worsened by induction therapies that can create additional hilar perivascular fibrosis. To compensate for these issues, minimally invasive techniques that mimic open surgical adaptions are useful.
Exploring the pericardial space often yields additional room for determining resectability and this was described in 2002 (23). Furthermore, this space is often free of adhesions and allows stapling on the proximal pulmonary artery or vein and even a limited portion of the left atrium so long as a thicker load is used. While occasionally needed for lobectomy, this is common for thoracoscopic pneumonectomy (24).
Large tumor anatomy can often disrupt the customary steps preferred by a minimally invasive lung surgeon. Better assessment of the proximal vessel and enabling of distal vascular control is also enabled by opening the interlobar fissure. Thus, if surgeons who prefer a fissure-last approach for routine lobectomy occasionally practice a tunnel-technique or similar method to open fissures they will be better prepared. Likewise, surgeons who like to finish with the bronchus need know how to divide the bronchus early to improve exposure when required.
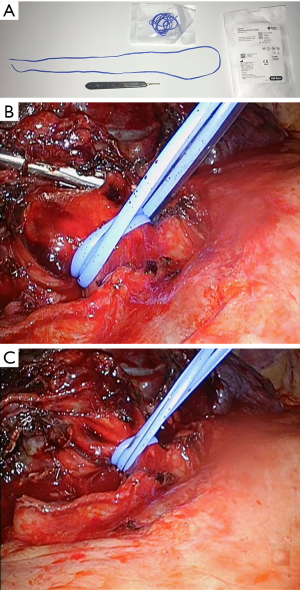
Finally, central PA dissection for the purpose of rapid clamping in the event of severe hemorrhage was a tenet of open surgery for chronic inflammatory conditions like tuberculosis resections. In a similar way, bleeding can be arrested by placing a vascular control device around the main pulmonary artery when dealing with tumors or other pathologies abutting the central vessels. This was described using a heavy silk double loop snare with a secondary loop to release the snare (25). Our technique is similar except that we prefer a silicone sling of extra length (762 mm, Aspen Surgical, Caledonia, MI) so that it can be doubled to distribute the occlusion forces more uniformly (Figure 6). The ends of this sling can exit one of the ports or be left intracorporeally with retrieval sutures in place for rapid control if hemorrhage occurs suddenly during tedious dissection.
T3 options
T3 tumors as defined by the AJCC 8th Edition are greater than 5 cm but less than 7 cm, involve chest wall, pericardium, phrenic nerve, or have separate satellite nodules in the same lobe. Tumors that are T3 based on satellite tumor nodules in the same lobe will not be discussed. Resection of tumors involving the chest wall is covered in a separate article. It should be noted that some carefully selected high-risk patients with chest wall invasion have been treated successfully at our center with preoperative radiation followed by intraoperative brachytherapy in order to avoid rib resection which generally increases operative risks close to that of pneumonectomy (26).
For tumors that are T3 based purely on size criteria, detaching the tumor from the hilar structures by performing a wedge resection if possible as previously described can facilitate dissection of more critical structures. Often this scenario will dictate stapling through very thick parenchymal tissue, requiring the thickest stapler load available.
Pericardium
For tumors invading the pericardium, thoracoscopic division of the pericardium is feasible and often presents fresh tissue planes not affected by tumor or adhesions. Thoracoscopic approaches for managing pericardial effusions have been reported dating back to the early 1990’s, and applying these techniques to open the pericardium when resecting a tumor is straightforward, with the exception that with oncologic resections there likely will be less pericardial fluid present (27). Care must also be taken to minimize the extent of pericardial resection, as reconstruction will be necessary to prevent catastrophic cardiac herniation which can result from a large pericardial defect.
Phrenic nerve
When tumor involves the phrenic nerve and requires en bloc resection, diaphragm paralysis will result and can increase postoperative morbidity from respiratory impairment. Published reports for thoracoscopic diaphragm plication for diaphragm paralysis and eventration describe suturing the diaphragm with interrupted or continuous sutures, or using stapling devices (28-30). These techniques can be applied at the time of resection, and have been described recently (31).
T4 options
With the 8th addition of the AJCC staging system, T4 tumors based on size >7 cm and nodules in ipsilateral lobes can be approached more easily using bilobectomy or pneumonectomy methods or the techniques described earlier in this article. On the other hand, minimally techniques for anatomically complex T4 tumors need schemes like that employed open and have been reported infrequently. The section will describe the major T4 challenges by type of anatomic invasion. In cases where there have been few reports of minimal invasive resections of these related organs for lung malignancy, reports of resections for other indications will be provided.
Two types of T4 classification will not be discussed in detail. First, the generic term of “Mediastinal” invasion has been difficult to classify because it can range from simple invasion of the fatty anterior mediastinal tissues to integral vascular, airway and spinal structures which will be addressed. Because it is a vague term and used infrequently, removal of “mediastinal pleural invasion” from the staging system has been adopted (32). Second is esophageal invasion which can be a superficial problem of the muscle treated by en bloc removal of the muscle leaving the mucosa intact. However, even this has a poor prognosis and a formal open or minimally invasive esophagectomy, while feasible in some circumstances, is rarely indicated because morbidity and mortality risks (33).
Diaphragm
Diaphragm resection for limited lung cancer invasion is relatively easy and can be performed with primary repair in many circumstances. Unfortunately, the depth of invasion relates to prognosis so wide areas of resection for lung cancer are unlikely to lead to long-term survival and should be avoided (34). Thoracoscopic diaphragm resection has been reported for the management of secondary thoracic malignancies and for mesothelioma (35-37).
Generally a running or interrupted permanent suture is used. A heavy braided non-absorbable stitch can be placed through the diaphragm and buttressed with absorbable or permanent pledgets if desired. Sutures can be placed with a standard laparoscopic needle holder or a suture enhancing device like the Endo-Stitch™ (Medtronic, Minneapolis, Minnesota, USA). Also, a knot enhancing device like the Ti-Knot (LSI Solutions, Victor, New York, USA) can also be helpful for anchoring sutures or patch material in difficult to tie locations like near the chest wall. Alternatively, a wound closing suture passer like the Carter-Thomason (CooperSurgical Inc., Trumbull, Connecticut, USA) can be used to deliver anchoring sutures through the chest wall to be tied extracorporeally allowing the knots to retract under the skin (37).
Spine
While not applied regularly for lung malignancy, there has been growing interest in the use of minimally invasive approaches to insert spine instrumentation to avoid the morbidity of thoracotomy. In some circumstances, VATS can be used to disconnect the lobe from it hilar attachments allowing it to be removed en bloc with the spine specimen. This can be done before or after the posterior laminectomy or limited posterior thoracotomy or other approach to remove the vertebral body and other spinal elements (38). Depending on the complexity and time required, sometimes a staged strategy is optimal with the neurosurgical team performing their work beforehand on another day of that hospitalization.
Central airway
Because suturing the airway for sleeve resections has been established for some time, the main impediment for more central airway tumors has been providing for cross field ventilation. This can be accomplished by delivering an airway through the utility port or through a separate stab incision. In situations where a carinal pneumonectomy has been required, a hybrid approach has been reported where the airway dissection, division, and reconstruction is performed using a thoracotomy and then the patient is flipped to allow a thoracoscopic pneumonectomy on the opposite side (39).
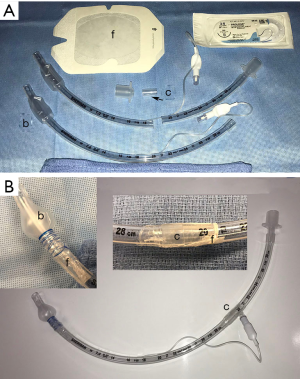
Limited distal tracheal resections have also been performed by thoracoscopic techniques (40). While the experience with such resections are limited, several technical points deserve mentioning. There needs to an endotracheal tube with sufficient length to ventilate the distal airways. Some authors have used a nasotracheal tube in the orotracheal position to achieve this length. We have fabricated a useful tube by starting with a standard 7 mm endotracheal tube. Another 7 mm tube cut just before the pilot tube can be used to increase the length sufficiently to intubate distal airways. Both tubes are connected by cutting off the internal flange of the circuit connector and reversing for a secure fit. Semipermeable intravenous catheter barrier film applied across the external connection provides additional security. Finally, a monofilament polypropylene suture can be wound around the distal balloon to reduce the balloon to a smaller size appropriate to a bronchus (Figure 7). Airways are reconstructed as in open operations with traction sutures such as 2.0 polypropylene and running barbed tieless sutures (3.0 for tracheal work) are increasing in popularity for minimally invasive approaches.
Cardiac and great vessel
Central vascular and heart invasions by tumors can be grouped depending whether or not they require cardiopulmonary support perfusion services during the key elements of the procedures. Recurrent nerve involvement generally means that there has been vascular invasion making reconstruction difficult or unlikely to augment survival, particularly when the aortic arch is involved. There may be some rare cases, however, where the preoperative imaging does not support that diagnosis and thoracoscopic inspection is reasonable to confirm vascular invasion.
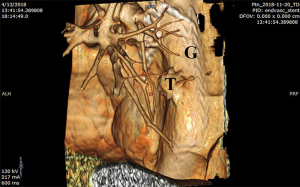
Cardiopulmonary bypass (CPB) may be avoided in some circumstances for certain T4 situations although it may be useful to have it available. The use of CPB, per se, does not seem to adversely affect long-term survival (41). Also, cell-saver technology is useful when severe bleeding is a reasonable possibility. Thoracic Endovascular Aortic Repair (TEVAR) is employed for situations where only a small portion of the thoracic aorta is vulnerable for injury during thoracoscopic resection. In those cases, a stent graft can be deployed preoperatively excluding the area that might be damaged during resection (42). Provided that there is no endoleak, a small portion of the aortic wall can be resected safely exposing the wall of the stent during the process. This technique has also been used to protect aortic integrity before delivering ablative high radiation doses to tumors juxtaposed to the vascular walls (Figure 8).
Similarly, if only a small portion of the left atrium is involved by tumors invading to the base of the pulmonary veins, an endoscopic surgical stapler can be used to remove this small portion of the heart (43). Generally, a size thicker than that used on vessels is chosen (3.5 mm or greater such as blue or purple). Limited invasion of the superior vena cava or the brachiocephalic vein can be managed by temporary occlusion if tolerated by the patient using maneuvers like head elevation to reduce cerebral edema (44). During occlusion the tumor can be resected and the vessel repaired by patch plasty performed through the utility incision. Vascular clamps, needle drivers and other useful instruments that are available in many operating rooms for endoscopic cardiac surgery can also be used for this type of repair.
In centers that are adept at both minimally invasive cardiac surgery and advance thoracoscopic lung tumor resection, it may be appropriate to combine the two with CPB strategies. Such indications may be limited particularly when there are well established competing approaches like median sternotomy that may be more practical (45). That stated, there are many frail patients in whom standard open approaches pose sufficient risks to consider adopting and adapting other new technologies provided oncology principles are maintained. In circumstances where there is tumor thrombus extending into the left atrium, total CPB may be needed to prevent catastrophic embolic complications. Anesthesiology team is critical in such cases to monitor factors like cannulation, cardiac performance and proper de-airing maneuvers.
Conclusions
Minimally invasive lung resections for tumors with advanced T-status are becoming more common as experience is accrued within centers of excellence and their reliabilities avoiding open surgery approaches 100%. As long as oncologic principles are followed, there probably are few absolute contraindications to attempt such resections. Conversion rates of 25% or more are to be expected but should be controlled without severe blood loss. Accordingly, proximal sling control of the main PA when it is at higher risk should be comfortable maneuver for surgeons. Tumors so large that a thoracotomy will be necessary for specimen extraction are a relative contraindication. In such cases, VATS may still be useful to establish resectability or provide additional useful vantage points for dissection if the tumor blocks the line of sight through the thoracotomy. Thus far, there have not been reports of increased local or systemic recurrences. That being said, operations that become prolonged because of tedious dissection or have needs for advanced support like cardiopulmonary bypass may be impractical. In our experience, the use of multiple retractors to set up exposure of bulky tissue, 3D camera optics, energy devices to control oozing and divide vessels too encumbered for stapling, and ability to adapt order of dissection with fissure opening as necessary are key aspects for success.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “VATS for Locally Advanced Lung Cancer”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.12.03). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. TLD served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Demmy TL, Yendamuri S, D'Amico TA, et al. Oncologic equivalence of minimally invasive lobectomy: The scientific and practical arguments. Ann Thorac Surg 2018;106:609-17. [Crossref] [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for non-small cell lung cancer by video-assisted thoracic surgery: Effects of cumulative institutional experience on adequacy of lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: Report of calgb 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-353; discussion 353. [Crossref] [PubMed]
- Decaluwé H, Petersen RH, Brunelli A, et al. Multicentric evaluation of the impact of central tumour location when comparing rates of n1 upstaging in patients undergoing video-assisted and open surgery for clinical stage i non-small-cell lung cancer. Eur J Cardiothorac Surg 2017; [Epub ahead of print]. [PubMed]
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of t status and n status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20; discussion 520-11.
- Pischik VG. Technical difficulties and extending the indications for vats lobectomy. J Thorac Dis 2014;6:S623-630. [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-273. [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Kamel MK, Nasar A, Stiles BM, et al. Video-assisted thoracoscopic lobectomy is the preferred approach following induction chemotherapy. J Laparoendosc Adv Surg Tech A 2017;27:495-500. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-976.e2. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Bertolaccini L, et al. Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: Analysis from multi-institutional national database. J Vis Surg 2017;3:160. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-413. [Crossref] [PubMed]
- Fan J, Yao J, Wang Q, et al. Safety and feasibility of uniportal video-assisted thoracoscopic surgery for locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:3543-50. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Dong S, Yang XN, Zhong WZ, et al. Comparison of three-dimensional and two-dimensional visualization in video-assisted thoracoscopic lobectomy. Thorac Cancer 2016;7:530-4. [Crossref] [PubMed]
- Yang C, Mo L, Ma Y, et al. A comparative analysis of lung cancer patients treated with lobectomy via three-dimensional video-assisted thoracoscopic surgery versus two-dimensional resection. J Thorac Dis 2015;7:1798-805. [PubMed]
- Liang H, Liang W, Lei Z, et al. Three-dimensional versus two-dimensional video-assisted endoscopic surgery: A meta-analysis of clinical data. World J Surg 2018;42:3658-68. [Crossref] [PubMed]
- Ibrahim M, Menna C, Maurizi G, et al. Impact of transcollation technology in thoracic surgery: A retrospective study. Eur J Cardiothorac Surg 2016;49:623-6. [Crossref] [PubMed]
- Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: Sorting through methods to prevent it. HPB Surg 2012;2012:169351 [Crossref] [PubMed]
- Kato M, Onishi H, Furugaki K, et al. New approach to complete video-assisted thoracoscopic lobectomy in t2 and t3 non-small cell lung cancer. Anticancer Res 2015;35:3585-9. [PubMed]
- Loscertales J, Jimenez-Merchan R, Congregado-Loscertales M, et al. Usefulness of videothoracoscopic intrapericardial examination of pulmonary vessels to identify resectable clinical t4 lung cancer. Ann Thorac Surg 2002;73:1563-6. [Crossref] [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: An 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Nakashima S, et al. How to clamp the main pulmonary artery during video-assisted thoracoscopic surgery lobectomy. Eur J Cardiothorac Surg 2007;31:129-31. [Crossref] [PubMed]
- Bourgeois DJ 3rd, Yendamuri S, Hennon M, et al. Minimally invasive rib-sparing video-assisted thoracoscopic surgery resections with high-dose-rate intraoperative brachytherapy for selected chest wall tumors. Pract Radiat Oncol 2016;6:e329-e335. [Crossref] [PubMed]
- Mack MJ, Landreneau RJ, Hazelrigg SR, et al. Video thoracoscopic management of benign and malignant pericardial effusions. Chest 1993;103:390S-393S. [Crossref] [PubMed]
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: A review of the literature. Ann Thorac Surg 2010;89:S2146-2150. [Crossref] [PubMed]
- Kara HV, Roach MJ, Balderson SS, et al. Thoracoscopic diaphragm plication. Ann Cardiothorac Surg 2015;4:573-5. [PubMed]
- Moon SW, Wang YP, Kim YW, et al. Thoracoscopic plication of diaphragmatic eventration using endostaplers. Ann Thorac Surg 2000;70:299-300. [Crossref] [PubMed]
- Takahashi Y, Miyajima M, Mishina T, et al. Thoracoscopic one-stage lobectomy and diaphragmatic plication for t3 lung cancer. J Cardiothorac Surg 2018;13:86. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Martini N, Yellin A, Ginsberg RJ, et al. Management of non-small cell lung cancer with direct mediastinal involvement. Ann Thorac Surg 1994;58:1447-51. [Crossref] [PubMed]
- Yokoi K, Tsuchiya R, Mori T, et al. Results of surgical treatment of lung cancer involving the diaphragm. J Thorac Cardiovasc Surg 2000;120:799-805. [Crossref] [PubMed]
- Yokoi K. combined resection of the chest wall and diaphragm in patients with lung cancer. Nihon Geka Gakkai zasshi 2016;117:301-7.
- Afandi O, Sutter O, Martinod E. Video-assisted diaphragmatic resection and reconstruction for intra-thoracic recurrence of hepatocellular carcinoma. J Visc Surg 2017;154:383-4. [Crossref] [PubMed]
- Demmy TL, Platis IE, Nwogu C, et al. Thoracoscopic extrapleural pneumonectomy for mesothelioma. Ann Thorac Surg 2011;91:616-8. [Crossref] [PubMed]
- Stoker GE, Buchowski JM, Kelly MP, et al. Video-assisted thoracoscopic surgery with posterior spinal reconstruction for the resection of upper lobe lung tumors involving the spine. Spine J 2013;13:68-76. [Crossref] [PubMed]
- Aim A, Almre I, Vanakesa T. Combined approach using uniportal video-assisted thoracoscopic surgery in left tracheal sleeve pneumonectomy. J Thorac Dis 2018;10:E584-6. [Crossref] [PubMed]
- Hung WH, Chen HC, Huang CL, et al. Thoracoscopic tracheal resection and reconstruction with single-incision method. Ann Thorac Surg 2018;106:e45-e47. [Crossref] [PubMed]
- Fu Q, Li QZ, Liang DG, et al. Early and long-term results of combined cardiac surgery and neoplastic resection in patients with concomitant severe heart disease and neoplasms. Chin Med J (Engl) 2011;124:1939-42. [PubMed]
- Otani S, Tsubochi H, Endo S, et al. Endovascular stent graft for surgical resection of lung cancer invading aortic arch: Report of a 79-year-old patient. J Vis Surg 2016;2:20. [PubMed]
- Xiao F, Bao T, Chen J, et al. Video-assisted thoracoscopic surgery in the treatment of non-small-cell lung cancer complicated with left atrial tumor thrombus. Thorac Cancer 2016;7:154-8. [Crossref] [PubMed]
- Xu X, Qiu Y, Pan H, et al. Resection of the sidewall of superior vena cava using video-assisted thoracic surgery mechanical suture technique. J Thorac Dis 2016;8:612-6. [Crossref] [PubMed]
- Langer NB, Mercier O, Fabre D, et al. Outcomes after resection of t4 non-small cell lung cancer using cardiopulmonary bypass. Ann Thorac Surg 2016;102:902-10. [Crossref] [PubMed]
Cite this article as: Hennon M, Demmy TL. VATS for advanced T status (large tumors, mediastinal invasion and vascular control). Video-assist Thorac Surg 2018;3:50.