Effective instruction by novel simulation technique
Introduction
During the last decades a plethora of papers have proved several advantages of minimally invasive surgery (MIS) to open surgery. For this reason, MIS has been adopted as usual approach in several surgical specialties (1-5).
In thoracic surgery, video-assisted thoracic surgery (VATS) is being increasingly recommended as standard approach for thoracic procedures due to the associated improved patient outcomes (2-4). In this sense, VATS must be considered the approach of choice in early stage lung cancer, but also should be taken into account in specific cases of advanced stage lung cancer (2,6).
However, 2018 ESTS Database annual report shows that VATS lobectomies performed in Europe are less than 38% in the last 5 years (7) This inconsistency could be explained because VATS can result a challenge for surgical trainees and staff members not accustomed to this approach carrying on important disadvantages to implement a VATS program.
Furthermore, the implementation of a new surgical technique can be time-consuming while developing and refining it (8-10). Early phases of learning curves in humans may involve important disadvantages for patients, but for those cases supervised by expert VATS surgeons. It has been concretely explained that an experienced thoracic surgeon consultant requires 30 lobectomies to be able to perform quality surgery. But this amount of cases, high up to 90 when we want to become efficient (11).
Besides, paramountly important is the number of cases treated in the institution. In those hospitals without VATS surgery every day learning curves can spread along lots of time. All these are important reasons for what we have implemented a realistic, reproducible and inexpensive VATS lobectomy simulation model (12).
Methods
Swine block
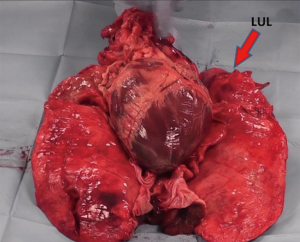
In our model we employ heart-and-lung tissue blocks from swine which are easily available in any pig slaughterhouse. The specimens come from slaughtered pigs for human consumption. Heart, both lungs, pericardium, trachea and oesophagus are included in the block Figure 1. Blocks are preserved freezed and slowly defrosted for 24 hours before their use. Fresh specimens have a better tissue quality, but it is logistically very difficult to have them fresh and dispose a session whenever needed. The cost of one of these swine blocks is around €1 in our setting. Additional materials needed are 150 to 200 mL of generic brand ketchup, some drops of red and blue food colouring, two syringes and two silk sutures to prepare de model. The complete material has an approximated total cost of €1.
Model preparation
We preserve all mediastinal structures from our blocks in order to work with an intact hilum that make surgery more realistic. In this way, the preparation of the model take place from inside of the pericardium in order to preserve extra-pericardium hilum for dissection.
In our institution left upper lobectomy (LUL) is the one performed in the ex vivo simulation model in all cases. LUL is the one chosen due to several reasons. Firstly, anatomy of left upper lobe is pretty similar to human anatomy, whereas right one loses realism because upper bronchus comes directly from trachea above carina and have 4 lobes. Secondly, LUL is the most difficult lobectomy to perform in humans because of the variability of arterial branches in number and position, so that it can help surgeons to improve their skills.
Simulated blood
We mix general brand ketchup with red food colouring to simulate oxygenated blood and ketchup with blue food colouring for non-oxygenated blood. Two 50 cc syringes with catheter tip are filled with both mixtures.
Filling pulmonary vascular beds
To carry on with the preparation, first of all, we place the model in a supine position. Pericardium is opened and great vessels exposed. We dissect left main pulmonary artery and upper left pulmonary vein intrapericardically and 0-silk sutures are passed around them. A half centimeter arteriotomy is then performed in the base of left pulmonary artery and it is filled with approximately 50 cc of the simulated non-oxygenated blood. Ketchup perfusion must be kept until a mild resistance is encountered, checking vessels distension. While we draw the syringe back, we must tie the silk in order to avoid ketchup leak. We must complete the same procedure with the vein, but in this occasion, we will make an atriotomy on the base of left atrial appendage, directing from there the syringe to the left upper vein.
Pulmonary vessels are then filled with red or blue-coloured-ketchup in order to let us know when vessels have been injured during dissection. Ketchup has an appropriate thickness to leak when vessels are injured, but also allows us to repair that injury and go on with our simulation.
After our block is prepared, we insert it in our simulation box, which can be considered from a black box to a 3D printed model of a thorax, customized with the incisions we will perform in a real patient. The specimen should be posed with left lung up in the theoretical way we will find it in a human being. All this model preparation takes around 10 minutes.
Lobectomy technique
Extra-pericardial dissection is always performed. Vein must be dissected and divided in first place. After that, first artery branch should be looked for, dissected and divided, followed by upper bronchus. Afterwards, the rest of arterial branches must be encountered and finally we will section the fissure. These steps can be carried out with mechanical staplers or manual sutures depending on our personal outcomes or economical sustainability.
In order to check usefulness of the program, we developed a project evaluating the impact of a simulation program in 4 staff thoracic surgeons and one trainee. Depending on expertise level, surgeons were divided into three groups: (I) high expertise VATS level (more than 100 VATS lobectomies) to which one surgeon belonged (expert group); (II) intermediate expertise VATS level (more than 30 VATS lobectomies) with three surgeons included (intermediate group); and (III) low expertise level (less than 30 VATS lobectomies) with one surgeon on it (beginner group).
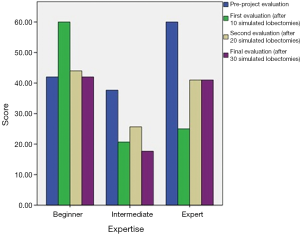
For six months, 5 surgeons performed 30 lobectomies in the ex vivo simulation program. All participants completed a pre-project evaluation, with re-evaluation after lobectomies number 10 and 20. A final evaluation was carried out (after complete 30 lobectomies performed). Evaluation results were calculated following an objective score system appraising time consuming and accuracy (Figure 2) (13). Maximum time allowed to complete lobectomy was 60 minutes. The examiner was the thoracic surgeon with the highest level of expertise in VATS resections in our team (more than 200 VATS lobectomies).
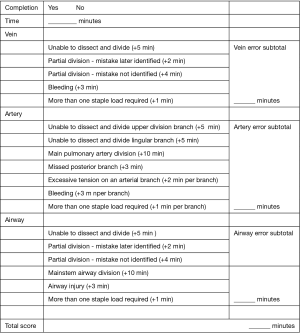
Evaluation scores were analysed with Wilcoxon test, comparing pretraining score with the one obtained after having completed 30 swine lobectomies. Improving percentage of each participant was also measured.
Results
Surgeons included in the project were four Board Certified Thoracic Surgeons and one Thoracic Surgery resident. Swine lobectomy number 1 (pre-project evaluation) was totally completed by all surgeons, although one in intermediate expertise level and the one of the high expertise level took the maximum time to complete it. Time needed for it was 42 minutes in beginner group, 37.7±20.7 minutes in intermediate group and 60 minutes in expert group.
Every participant completed post-project evaluation lobectomy, with medium scores of 42 minutes in beginner group, 17.7±4.2 minutes in intermediate group and 41 minutes in expert one. Scores obtained in second and third evaluation are represented in Figure 3. Although the differences between pre-project evaluation and post-project scores were not significant, the improving percentage was 0% (beginner group), 54.3% (intermediate group) and 31.7% (expert group).
After 30 simulated sessions performed like previously described, the great improvement was observed in the intermediate level. Experienced VATS surgeon did not improve results significantly. And trainee group did not improve either; this could be explained by the fact that this group may need a larger number of cases before showing an important improvement. To this point, we must assume that this model is not focussed in very experienced surgeons, but it can result of relevant help to those surgeons already performing VATS lobectomies with not a high expertise level (less than 100 cases).
Our service started 4 years ago (in 2014) a VATS program. Two years later, the ex vivo simulation program was implemented. To evaluate the influence of the simulation program on our VATS program we divided the last four years in three lapses of time: (I) VATS pre-simulation program (from 1st January 2014 until 31st December 2015); (II) start-up of simulation program (from 1st January 2016 till 31st December 2016); (III) consolidation of simulation program (from 1st January 2017 till 31st December 2017). During this period, a total of 736 consecutive patients submitted to anatomical lung resections were included. Surgical approach (VATS or thoracotomy), conversion rate and postoperative complications (according to Clavien-Dindo classification) were analyzed.
Three hundred and seventeen patients were included in pre-simulation period, 204 in the startup period and 215 in consolidation period. VATS resection rate was 29.3% before simulation program, and high up to 46.1% after implementation of ex vivo simulation program. When the simulation program was consolidated, VATS anatomical resection rate was 71.2% of total. Analyzing individually each surgeon, we found out that whereas in the first period just one of the five surgeons of our team reached 50% of VATS resections, in the third period all the surgeons reached 45% and three of them got over 80%. Global results are shown in Table 1.

Full table
VATS complication rate was lower than global complications, and VATS mortality rate was 0% in every period.
So, in our unit, ex vivo simulation program helped to establish and consolidate a VATS program in a safety way with low morbidity and conversion rates. All the surgeons in the unit were involved in the program and improvement of VATS ratio can make us assume the utility of simulation. It also helps to standardize VATS technique and optimize surgical times and procedure.
Discussion
Nowadays, there are also several systems of virtual-reality endoscopy training. These technological simulations have shown improvement on knowledge, skills and attitude in surgeons (14,15). This kind of simulators make preparation easier than the ex vivo simulation model. However, if we want to test technical specific skills different to the usual ones, or to test new instruments, we should reprogram our computer or change the software, meaning time-consumption and money investment.
On the other hand, whenever we want to apply this in an ex vivo model, we just have to carry on it. Up to now, no studies comparing videothoracoscopic virtual reality simulators and ex vivo simulators have been compared. It would be interesting to determine efficiency, reproducibility, feasibility and costs between both kind of simulation. Next years, a mixed simulation program with ex vivo and virtual reality may likely show advantages to improve different stages of VATS training.
Conclusions
To sum up, the addition of an ex vivo simulation program to a VATS program for anatomical lung resections can accelerate the implementation of the program (increasing VATS rate quickly), improve safety (decreasing morbidity and conversion rates) and makes VATS surgery available for every surgeon.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yoshihisa Shimada) for the series “Current Status of Surgical Simulation in Video-assisted Thoracic Surgery and Robot-assisted Thoracic Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.09.04). The series “Current Status of Surgical Simulation in Video-assisted Thoracic Surgery and Robot-assisted Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jensen K, Petersen RH, Hansen HJ, et al. A novel assessment tool for evaluating competence in video-assisted thoracoscopic surgery lobectomy. Surg Endosc 2018;32:4173-82. [Crossref] [PubMed]
- Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer 2016;100:114-9. [Crossref] [PubMed]
- Long H, Tan Q, Luo Q, et al. Thoracoscopic Surgery Versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. Ann Thorac Surg 2018;105:386-92. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- The ESTS Database Report Silver Book 2017. Available online: http://www.ests.org/collaboration/database_reports.aspx
- Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [Crossref] [PubMed]
- Moon MR. Technical skills assessment in thoracic surgery education: we won't get fooled again. J Thorac Cardiovasc Surg 2014;148:2497-8. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught?. Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Depypere L, De Jonghe L, Peetermans W, et al. Does the implementation of European Working Time Directive (EWTD) have an effect on surgical training in a Flemish teaching hospital network? Acta Chir Belg 2014;114:299-303. [Crossref]
- Jimenez M, Gomez-Hernandez MT. Teaching video-assisted thoracic surgery lobectomy-using an ex vivo simulation model. J Vis Surg 2017;3:34. [Crossref] [PubMed]
- Tong BC, Gustafson MR, Balderson SS, et al. Validation of a thoracoscopic lobectomy simulator. Eur J Cardiothorac Surg 2012;42:364-9; discussion 369. [Crossref] [PubMed]
- Cook DA, Hatala R, Brydges R, et al. Technology-enhanced simulation for health professions education: a systematic review and meta-analysis. JAMA 2011;306:978-88. [Crossref] [PubMed]
- Gallagher AG, Neary P, Gillen P, et al. Novel method for assessment and selection of trainees for higher surgical training in general surgery. ANZ J Surg 2008;78:282-90. [Crossref] [PubMed]
Cite this article as: Jimenez MF, Fuentes-Gago MG, Gomez-Hernandez MT. Effective instruction by novel simulation technique. Video-assist Thorac Surg 2018;3:42.