
Video-assisted thoracoscopic surgery in the management of malignant pleural disease
Introduction
Malignant pleural disease includes the following conditions either the pleura may represent the site of the primary tumour [malignant pleural mesothelioma (MPM)] or a metastatic site from proximal or distant organs. The disease can be categorized by whether the lung will expand or whether it is entrapped. It may present as diffuse or nodular pleural thickening, with or without a malignant pleural effusion (MPE).
The approximate annual incidence of MPEs in the United States is 200,000 (1) with an overall incidence of both malignant and paramalignant effusion up to 50% in patients with thoracic or extra-thoracic malignancies (2). In Europe, the frequency of MPE is estimated at nearly 400,000 patients per year and ca 50,000 new MPE are diagnosed in the UK every year.
MPE is traditionally attributed to a defect in the pleural fluid drainage but the lymphatic block cannot explain the co-existent immune-response. Recent evidence in fact highlights the pivotal role of an interaction of the host immune-system with the cancer cells which triggers the extravasation and may be modulated by especially vascular endothelial growth factor-A (VEGF-A). Immune-cells appear self-sustaining thus increasing the vascular permeability, cancer cell transmigration, and angiogenesis. These factors offer potential inhibitory targets for the pleural effusion mechanism (3,4).
Patients with non-small cell lung cancer (NSCLC) with pleural seeding and MPE (M1a) have poor outcomes, with a median survival time (MST) of 11.5 months (5). Thus, when pleural involvement is an accidental intraoperative finding even if localized the current consensus favours open-close surgery followed by chemotherapy or targeted therapy for stage IV disease. There is no evidence to date that the resection of the primary tumour has a favourable impact on the prognosis (6).
The role of video-assisted thoracoscopic surgery (VATS) in malignant pleural disease
Surgical treatment of malignant pleural diseases is considered palliative (7) as the indication is usually advanced disease associated with significant morbidity. Minimally invasive approaches must be offered as first line treatment since they have the important advantages of causing less surgical trauma, reduced post-operative pain.
The accuracy of VATS for diagnostic purposes is undebatable with a 95% sensitivity rate for malignancy and its effectiveness in preventing effusion recurrence by facilitating chemical pleurodesis has been widely recognized (8). Most of the evidence for therapeutic VATS pleural surgery is for primary MPM. In the treatment of metastatic NSCLC therapeutic VATS is concerned mainly in the treatment of infected malignant effusion.
We will consider the role of VATS in the management of malignant pleural disease in the following processes (Table 1): (I) chemical pleurodesis or parietal pleurectomy if the lung expands; (II) indwelling pleural catheter (IPC) insertion or visceral pleurectomy in the entrapped lung; (III) debridement/decortication for the malignant pleural empyema.
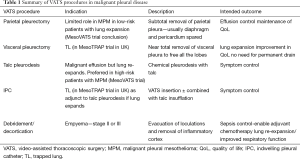
Full table
Lung seen to expand at VATS
VATS talc pleurodesis
VATS represent an invaluable diagnostic tool particularly in pleural Mesothelioma where the low diagnostic rate of pleural fluid cytology alone (20–32%), especially in the sarcomatoid type, makes of it an unreliable technique that needs to be supported with supplementary techniques as immunohistochemistry (9,10). Video-assisted thoracoscopy for the diagnosis of malignant mesothelioma has been also recommended as the technique of choice, allowing extensive inspection of the pleura and the taking of multiple and large biopsies that include subpleural tissue for the histological assessment of invasion (11). If the lung will expand, in order to achieve pleural symphysis talc poudrage is usually performed using 4–8 grams of sterile talc powder insufflated into the pleural space. Talc has been shown to have better efficacy than other sclerosants to prevent recurrence with a success rate of 81% to 100%. A recent study investigated the success rate and complications of low dose (5 gr) compared to high dose talc (10 gr) in patients with MPE, showed how the recurrence of pleural effusion (at 6 weeks) or immediate morbidity (48 hours) were statistically not significant between the two groups (P>0.05). Adult respiratory distress syndrome (ARDS) was not seen in either group (12).
VATS parietal pleurectomy
As the parietal pleura seems to be most important in the physiologic mechanism of fluid production and absorption because of its proximity to lymphatic openings to the pleural cavity by and micro-vessels (13) the procedure of parietal pleurectomy can achieve successful recurrence free rates >90% at 12 months (14) with relatively lower risk then open surgery (15).
VATS parietal pleurectomy aims to produce fusion of the pleural cavity by using a similar technique to that used in pneumothorax surgery. Under general anaesthesia with single-lung ventilation, the first step is to evacuate the pleural effusion under vision and to assess whether the lung will re-expand with ventilation or whether it is entrapped which would contraindicate a parietal pleurectomy. The parietal pleurectomy is started from the anterior thoracoscopic access port and continued posteriorly down to the mediastinum and the costophrenic angle by blunt dissection of the extrapleural plane. The pleura over the pericardium and the diaphragm is usually spared but the remainder of all the visible foci of carcinomatosis are removed. Nevertheless, the adhesiolysis and debridement of pockets of effusions will eventually release the lung parenchima. At the end of the procedure the lung re-expansion is assessed. One or two chest drains are placed in the apex of the lung and at the base respectively and suction is applied.
Previous early reports had shown the feasibility and a better survival rate following a VATS debulking pleurectomy-decortication in advanced disease when compared to a thoracoscopic biopsy alone. In a retrospective study on 64 patients with MPM investigating the operative outcome of pleurectomy as a palliative treatment in patient unsuitable for extrapleural pneumonectomy the results showed that patients undergoing debulking of the tumour, drainage of the effusion, decortication, re-expansion of the lung, and pleurodesis had an overall median survival of 21.7 months (with a range of 1.4–52.7 months) when of epithelial histology versus 5.8 months for the sarcomatoid or mixed type (P=0.0001) (16). An UK cohort study comparing the actuarial survival of 79 patients with advanced MPM undergoing VATS pleural biopsy or VATS pleurectomy decortication showed feasibility and a significant improvement in survival in the debulking arm (127 vs. 416 d) (17).
The prognostic significance of video-assisted thoracoscopic partial pleurectomy (VAT-PP) compared to the standard approach with VATS talc pleurodesis offered to control the pleural effusion secondary to MPM has been assessed in the controlled randomised trial, the MesoVATS trial (18). The results showed that overall survival (OS) rates were not significantly different between the treatment groups and the VAT-PP approach was associated with increased side-effects related to the surgical procedure i.e., air leak and extended hospital stay and was therefore found to be economically disadvantageous. OS at 6 months was in fact estimated to be 78% in the VAT-PP group and 80% in the talc pleurodesis group, and at 1 year was estimated to be 52% and 57% respectively. Since the OS was the main outcome analyzed for the two groups in the study the benefits of the improved quality of life (QoL) were not sufficiently outlined (19). However, subgroup analysis showed that for patients with a favourable European Organization for Research and Treatment of Cancer (EORTC) prognostic score not only was OS at 6 months 88% but that there was significant effusion control and QoL improvement for up to 12 months. The overall impression created by the publication of the MesoVATS trial was that there is no role for VATS-PP as there was no survival benefit over talc pleurodesis. At 1 year, OS was 37% in high-risk patients in the VAT-PP group and 53% in high-risk patients in the talc pleurodesis group, and 63% and 61% in the low-risk group for VAT-PP and talc pleurodesis, respectively. There remain, however, doubts surrounding the heterogeneity in surgical method (variable degree debulking in the experimental arm, medical and surgical thoracoscopy) and the patient population (different subtypes and stages of disease) which mean that the procedure may still have a role in selected patients. QoL of patients with malignant effusions should be evaluated with regard to those symptoms that are related to the effusion itself. Relief of dyspnea remains the primary objective for most patients. Ideally, therapy should minimize discomfort, as well as limit hospitalization time, in these patients with an often-limited life span. However, an important aspect in any treatment is prevention of reoccurrence of the symptomatic effusion (20).
While VATS-PP may be justified in the better prognostic group in the context of metastatic pleural involvement from cancers of non-pleural origin, these cytoreductive strategies are not routinely offered due to the usual concomitant detrimental effect of systemic therapies on patient’s condition and the poorer life expectancy (21).
Entrapped lung seen at VATS
VATS placement of an IPC
An IPC is a silicone tube that is placed into the pleural cavity, tunnelled subcutaneously with a small (pro-fibrotic) cuff, with the other end exiting the patient with a one-way valve. Once tunnelled beneath the skin into the pleural cavity it can remain in place indefinitely, allowing easy drainage at home or in an ambulatory setting, by patients and their caregivers with minimal training. Management of symptoms as an outpatient allows patients to maintain control over their lives and minimizes the time the spent in the hospital (22).
There is increasing evidence that IPC are safe and effective in managing patient symptoms and improving the QoL median hospital length of stay for VATS pleurodesis is estimated as of 4 to 5 days, whereas an IPC is often inserted as a day-case surgical procedure. In addition, chemical pleurodesis requires sufficient body inflammatory response to fuse the visceral and parietal pleura in a short period of time. The tunnelled pleural catheter (TPC) procedure instead does not require this limitation and can be used in a wider set of patients (23).
In the TIME2 trial, an unblinded randomized controlled trial comparing IPC to talc pleurodesis, the IPC was inserted on an outpatient basis outpatient draining immediately a large amount of fluid, drainage was advised for 3 times weekly or as required for relief of dyspnoea. It showed how the dyspnoea improved in both groups, with no significant difference in the first 42 days (24). The improvement in dyspnoea and QoL in patients treated with IPC is comparable to talc pleurodesis up to 6 months, after which IPC may be superior in relieving dyspnoea and with the potential of autopleurodesis (25). Autopleurodesis is considered completely achieved when a decline in the amount of pleural fluid is observed (less than or equal to 50 mL on three consecutive attempts of drainage), absent or very minimal pleural effusion on the chest X-ray (CXR) (blunting <25% of the chest), and absence of symptoms. Spontaneous pleurodesis can develop in 40–70% of patients with IPC in situ, which permits catheter removal.
A rapid pleurodesis procedure, using the combination of thoracoscopy guided talc delivery for pleurodesis with TPC insertion at the same procedure takes advantages of both management strategies and minimizes some disadvantages. This method has previously been shown in two series to decrease hospital length of stay (mean 1.8–2 days), and duration of TPC use (mean 8–10 days) measured by time to pleurodesis while significantly improving dyspnoea and QoL in patients with MPE. It is to be considered an optimal management should the costs not be considered. The OPTIMUM trial is designed to determine whether full outpatient management of MPE with an IPC and pleurodesis improves QoL compared with traditional inpatient care with a chest drain and talc pleurodesis (26).
Nevertheless, catheter related complications can occur and include wound site infection, empyema, blocked or dislodged IPC, leakage around the catheter, pain or severe discomfort. Catheter tract metastases (CTM) although not uniformly defined in the different studies are new, solid chest wall lesions over the IPC insertion site and/or the tunneled subcutaneous tract. The reported incidence of procedure-tract metastases ranges in available literature from <1% to 10% and MPM seems the most predisposing cancer accounting for the majority of cases of IPC-related CTM (27).
Moreover, the presumed catheter and/or cancer induced fibrin deposition within the pleural cavity, along with the pleural symphysis resulting from the continuous drainage, can unfavorably induce septations and loculations, thus limiting effective IPC drainage. Hence, breathlessness in absence of pleural infection can be explained by the residual effusion. IPC-related symptomatic loculations are reportedly present in 6–14% of IPC-treated patients (28), and typically occurs at about 2 months after IPC insertion (27).
Guidelines have advocated the use of IPCs in those patients with MPE that have failed pleurodesis or in those with trapped lung (TL) (unsuitable for pleurodesis). IPCs offer long-term access to the pleural cavity, they represent ideal portals for local drug delivery with the potential of being an acceptable compromise in patients who wouldn’t be fit for a major operation. In a case review from 6 UK Centres aiming at estimating the survival in cases of pleural infection treated with IPC for MPE the rate of pleural infection was calculated as being the 3.6%. Surprisingly the study showed that patients with mesothelioma or lung cancer associated with pleural infection outlived the cohort without a pleural infection with an almost doubled MST (753 vs. 339 d for mesothelioma, 138 vs. 74 d for lung cancer) hypothesizing a trigger role for the pleural infection in stimulating an immune response (29).
One advantage of VATS over percutaneous image-guided insertion of IPC is that the surgical operator is able to proceed to other thoracic surgical options, if appropriate, at the time of the thoracoscopy. In particular, on the basis of the intra-operative assessment of the extension of the pleural tumour involvement and the entity of the lung potential of expansion the IPC can be inserted at the end of the procedure. In a study on 116 patients with proven MPE a VATS technique, under general anaesthesia for fit patients (41/116), was preferred in those whose history or radiology wasn’t obviously suggestive of TL.
VATS visceral pleurectomy
In advanced malignant pleural disease, the lung may become entrapped by a thickened visceral pleural rind of tumour which prevents its expansion causing underlying collapse and respiratory compromise. Therefore, the symptoms of dyspnoea are usually compounded by ventilation-perfusion mismatch within the entrapped lobe or lobes. This progressive disease process results in dyspnoea in most cases which can affect significantly the patients’ QoL. The lung re-expansion achieved as result of a successful decortication can successfully impact on the hypoxia and ventilation perfusion mismatch. The visceral pleurectomy will be carried out one anatomical layer lower than in a decortication for empyema with an incision of the visceral pleura with endoscopic shears which allows to suspend an edge with artery forceps and starting the blunt dissection from the underlying parenchyma with a blunt dissector. Intermittent continuous positive airway pressure can be applied to the operative lung to facilitate the dissection. The goal is to free all the lobes allowing adequate lung expansion and apposition of the parenchyma against the chest wall.
The ideal treatment of MPE should include adequate and enduring relief from symptoms (in particular dyspnoea), minimize hospitalization, and reduce adverse effects. Any planned treatment should balance the therapeutic benefit provided against the required period of convalescence for a disease with a limited life expectancy. As per any other debulking techniques the visceral pleurectomy aims for achieving therapeutic and palliative effects thanks to its potential to offer cytoreduction with the presumptive benefit of delaying tumour progression and prolonging survival (30). In presence of recurrent pleural effusion and thickened cortex the benefits of VATS visceral pleurectomy in relieving the TL in metastatic adenocarcinoma and MPM have been shown since early reports (31).
In a prospective cohort study enrolling 51 patients presenting with malignant meosthelioma (MM) undergoing palliative surgical debulking relief of chest wall pain was observed too. And the authors suggest that intercostal nerve compression could be the explanation. The author reports 78.4% of symptom control at 6 weeks (P value =0.01) and 70.6% and 41.2% at 3 and 6 months respectively. Patients with epithelial cell type and no weight loss were significantly more likely to retain symptomatic control than those with neither of these features (P<0.01) (32).
The recent randomized AMPLE trial failed to show any significant improvement in terms of breathlessness relief in the IPC arm over talc pleurodesis. The question of whether VATS visceral pleurectomy is more effective than a continuous drainage of the pleural effusion with an IPC is being addressed in the MesoTRAP trial (25).
This multicentre pilot clinical trial has been designed to be a preliminary study to a full Phase III study where the most effective management of a “TL” will be determined. It aims at randomizing 38 patients with TL and pleural effusion due to MPM who will be allocated in a 1:1 ratio to either video-assisted thoracoscopic partial visceral pleurectomy/decortication (VAT-PD) or IPC. The main inclusion criteria for the eligibility in the trial are: confirmed MPM, a “clinically significant TL requiring intervention in the opinion of the clinical team”, presence of pleural effusion (following re-accumulation), and fitness to undergo VAT-PD.
The primary outcome measure is the improvement of breathlessness confirming; the main purpose of non-radical treatments as symptomatic relief. The secondary outcomes include changes in chest pain, the assessment of post-procedure QoL according to two different questionnaires (EQ-5D-5L and EORTC QLQC30) and survival at 30 days and 12 months post-randomization.
VATS debridement/decortication
A loculated malignant effusion may become secondarily infected, especially if multiple percutaneous drainage interventions or an IPC device have been attempted. VATS debridement is sufficient if the underlying lung will expand but VATS decortication of the inflammatory cortex may be successful even if the lung is entrapped (see above). The resolution of ongoing pleural infection is important if the patient is to be considered for cytotoxic chemotherapy.
In a recent single-centre review of 561 patients with initial diagnosis of empyema, negative pre-operative cytology, absence of radiological or clinical features, 35 patients (6.2%) had a post-operative histological diagnosis of malignancy. Two third of the patients were treated by VATS approach (33). The role of VATS has been widely described in benign simple or complicated empyema (34). A recent meta-analysis seems showing that video-assisted thoracic decortication (VATD) might be comparable or even better than open thoracic decortication in terms of operative time, postoperative hospital stays, chest tube duration, prolonged air leak rate, morbidity and mortality (35). But the literature lacks studies on the application of video-assisted thoracic surgery in malignant empyema. Empyema post VATS lung resection for lung cancer are rarely reported though. Only 1 (0.1%) case of empyema over 139 cases of pulmonary complications following VATS lobectomy for lung cancer was reported in a recent retrospective study where no mention of the treatment was made (36).
Conclusions
VATS is designed to reduce chest wall trauma, preserve respiratory muscle function and therefore expedite recovery. Nowhere is this more important than in those patients with a limited prognosis with advanced malignant pleural disease. VATS also allow for therapeutic manipulation of the pleural environment including dissection techniques aimed at symptom control by direct tumour debulking.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.08.05). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Light RW. Pleural Diseases. Philadelphia: Lippincott Williams & Wilkins, 2013.
- Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017;3:85. [Crossref] [PubMed]
- Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012;186:487-92. [Crossref] [PubMed]
- Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep 2013;15:207-16. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg 2013;17:407-12. [Crossref] [PubMed]
- Whitworth JM, Schneider KE, Fauci JM, et al. Outcomes of patients with gynecologic malignancies undergoing video-assisted thoracoscopic surgery (VATS) and pleurodesis for malignant pleural effusion. Gynecol Oncol 2012;125:646-8. [Crossref] [PubMed]
- Luh SP, Chen CY, Tzao CY. Malignant pleural effusion treatment outcomes:pleurodesis via video-assisted thoracic surgery (VATS) versustube thoracostomy. Thorac Cardiovasc Surg 2006;54:332-6. [Crossref] [PubMed]
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [Crossref] [PubMed]
- Rakha EA, Patil S, Abdulla K, et al. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol 2010;38:874-9. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Jha RS. Low Dose vs High Dose Talc Pleu-rodesis in Malignant Pleural Effusion. Eur Respir J 2011;38.
- Ried M, Hofmann HS. The treatment of pleural carcinosis with malignant pleural effusion. Dtsch Arztebl Int 2013;110:313-8. [PubMed]
- Kara M, Alzafer S, Okur E, et al. The use of single incision thoracoscopic pleurectomy in the management of malignant pleural effusion. Acta Chir Belg 2013;113:270-4. [Crossref] [PubMed]
- Fry WA, Khandekar JD. Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol 1995;2:160-4. [Crossref] [PubMed]
- Neragi-Miandoab S, Richards WG, Sugarbaker DJ. Morbidity, mortality, mean survival, and the impact of histology on survival after pleurectomy in 64 patients with malignant pleural mesothelioma. Int J Surg 2008;6:293-7. [Crossref] [PubMed]
- Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314-20. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Petrov R, Bakhos C, Abbas AE. Management of Malignant Lung Entrapment, the Oncothorax. Thorac Surg Clin 2018;28:81-90. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Paiano S, et al. Indwelling Pleural Catheters: A Clinical Option in Trapped Lung. Thorac Surg Clin 2017;27:47-55. [Crossref] [PubMed]
- Sudharshan S, Ferraris VA, Mullett T, et al. Effectiveness of tunneled pleural catheter placement in patients with malignant pleural effusions. Int J Angiol 2011;20:39-42. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Rintoul RC, Tod A, Sivasothy P, et al. A feasibility study of indwelling pleural catheter versus VAT pleurecotomy for trapped lung in mesothelioma. Available online: http://imig2016.org/wp-content/uploads/2016/04/ iMig-2016-Abstract-Book.pdf
- Sivakumar P, Douiri A, West A, et al. OPTIMUM: a protocol for a multicentre randomised controlled trial comparing Out Patient Talc slurry via Indwelling pleural catheter for Malignant pleural effusion vs Usual inpatient Management. BMJ Open 2016;6:e012795 [Crossref] [PubMed]
- Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res 2016;3:e000123 [Crossref] [PubMed]
- Banka R, Terrington D, Mishra EK. Management of Septated Malignant Pleural Effusions. Curr Pulmonol Rep 2018;7:1-5. [Crossref] [PubMed]
- Bibby AC, Slade GC, Morley AJ, et al. S117 Survival In Patients With Malignant Pleural Effusions Who Developed Pleural Infection: A Retrospective Case Review From 6 Uk Centres. Thorax 2014;69:A63. [Crossref]
- Rathinam S, Waller DA. Pleurectomy decortication in the treatment of the "trapped lung" in benign and malignant pleural effusions. Thorac Surg Clin 2013;23:51-61. vi. [Crossref] [PubMed]
- Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg 1997;11:210-3. [Crossref] [PubMed]
- Martin-Ucar AE, Edwards JG, Rengajaran A, et al. Palliative surgical debulking in malignant mesothelioma. Predictors of survival and symptom control. Eur J Cardiothorac Surg 2001;20:1117-21. [Crossref] [PubMed]
- Valtzoglou V, Chowdhry MF; Roman M, et al. Poster 98: Pleural malignancy presenting as empyema thoracis. Review of a thoracic centre’s experience. SCTS Annual meeting 2017. Available online: https://www.myeventflo.com/event-lecture.asp?lectID=12016
- Hajjar WM, Ahmed I, Al-Nassar SA, et al. Video-assisted thoracoscopic decortication for the management of late stage pleural empyema, is it feasible? Ann Thorac Med 2016;11:71-8. [Crossref] [PubMed]
- Pan H, He J, Shen J, et al. A meta-analysis of video-assisted thoracoscopic decortication versus open thoracotomy decortication for patients with empyema. J Thorac Dis 2017;9:2006-14. [Crossref] [PubMed]
- Wang S, Li X, Li Y, et al. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis 2017;9:5143-52. [Crossref] [PubMed]
Cite this article as: Luciano G, Waller DA. Video-assisted thoracoscopic surgery in the management of malignant pleural disease. Video-assist Thorac Surg 2018;3:37.


