
Advanced VATS resections: radiation therapy and VATS
Introduction
A history of both induction therapy in general as well as specifically previous radiation therapy is a risk factor for morbidity and mortality after major lung resection. However, both prospective and retrospective studies have shown that thoracotomy and major lung resection after induction treatment is feasible. Performing major lung resection with video-assisted thoracoscopic surgery (VATS) after induction therapy was initially felt to be contraindicated in the early period of VATS use, but small retrospective studies have shown the feasibility of using VATS for major lung resection even after induction treatment. Depending on the specific clinical scenario and, perhaps most importantly, a surgeon’s experience with complex VATS lung resections, the use of VATS can be a safe and oncologically appropriate option for a patient after radiation. This manuscript will review the role and risks of major lung resection after previous radiation via both thoracotomy and VATS, as well as specific considerations and techniques that may be appropriate when performing VATS after radiation therapy.
Role of major lung resection after induction therapy
Clinical scenarios where surgery is indicated after radiation therapy
Performing major lung resection after radiation therapy is relatively uncommon. Induction chemotherapy or radiation therapy was used in only 1,801 (6.5%) of 27,844 patients who underwent lung resection in the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database over the time period 2012–2014 (1). However pulmonary resection after radiation therapy may be appropriate in several scenarios (2). Induction therapy prior to surgical resection is typically planned for patients with stage IIIA NSCLC due to N2 nodal involvement who are considered resectable at the time of diagnosis (3). Prospective, randomized trials have demonstrated a benefit to induction chemotherapy over primary surgery for these patients (4-6). Although the combination of induction chemotherapy with radiation therapy and then surgical resection for stage IIIA disease is feasible but has not been shown in randomized trials to improve survival, a survey of North American thoracic surgeons showed that most (70%) surgeons consider the most appropriate neoadjuvant therapy for stage III NSCLC to be chemotherapy combined with radiation (7-9). Most (80%) of these surgeons preferred a pre-surgery radiation dose of 45 Gy, while 19% reported using 66 Gy radiation and a very small minority utilized an even higher dose of radiation. Induction chemotherapy and radiation therapy prior to surgical resection is also typically pursued in patients with locally advanced superior sulcus tumors (T3 or T4) and N0 or N1 disease (10,11).
Other patients with locally advanced lung cancer may be treated with chemoradiotherapy when an initial resection is not technically feasible or does not appear likely to offer a survival benefit over non-operative therapy (2). These patients may have been initially deemed unsuitable for surgery for oncological or medical factors, such as initial clinical overstaging, patient refusal to consider surgery, poor lung function, or significant co-morbidities, only to have the patient or their oncologists reconsider that assessment after their initial course of therapy (12). Although uncommon, surgeons may consider resection after chemoradiation in these situations when patients have local or regional residual disease (3). Patients are also referred for major pulmonary resection to treat conditions such as necrotic lung or lung abscess refractory to medical therapy after definitive chemotherapy or radiation therapy (13). Finally, patients previously treated with definitive radiation therapy for a malignant process may develop a new metachronous primary malignancy for which surgery is deemed the optimal therapy.
Risks of surgery after radiation
Several early reports identified increased morbidity and mortality after the use of induction chemotherapy and radiation therapy prior to major lung resection (14,15). In particular, mortality is significantly higher when patients undergo pneumonectomy compared to lobectomy after induction therapy (7,15-17). Advances in surgical and perioperative care have likely reduced the potential impact of induction chemotherapy alone, as subsequent reports have shown no significant difference in mortality or morbidity in patients receiving surgery alone versus pre-operative chemotherapy followed by surgery for non-small cell lung cancer (18-20). Despite the advances and recent studies, induction therapy likely does generally confer at least some increased surgical risk. A report from the STS General Thoracic Surgery Database that reviewed 4,979 lobectomies performed between 2002 and 2006 showed induction therapy was a risk for a prolonged length of hospital stay, considered a surrogate for morbidity (21). An even more recent study of 27,844 patients who underwent lung resection in the STS General Thoracic Surgery Database over the more recent time period 2012–2014 continued to show that induction therapy was an independent risk factor for both major morbidity [odds ratio (OR) 1.2] and mortality (OR 1.51) (1). Risks that may be higher after induction therapy include bleeding during dissection of hilar structures that have extensive inflammatory or fibrotic radiation changes, bronchopleural fistula due to reduced bronchial tissue viability, and respiratory failure (22,23).
Results after lung resection via thoracotomy after radiation
Multiple studies have reported results of performing thoracotomy and major lung resection after previous treatment with radiation, typically also with chemotherapy (7,14-17,24-31) (Table 1). Early experience generally involved significant morbidity and mortality. Fowler et al. reported that one patient among six who had lobectomy had adult respiratory distress syndrome (ARDS) and three of seven pneumonectomy patients had a perioperative death when surgery was performed after 60 Gy radiation and concomitant chemotherapy (15). Bonomi et al. reported that 6 of 16 patients who had received split-course thoracic radiation (total 40 Gy) and chemotherapy followed by a thoracotomy had serious postoperative complications, including one death form ARDS, two bronchopleural fistulas, and one pneumonia (14). Deutsch et al. reported 3 perioperative deaths among 16 patients (19% mortality) with clinically staged IIIA non-small cell lung cancer who received induction chemotherapy and radiation therapy (60 Gy) and then thoracotomy (6 pneumonectomies) (16).
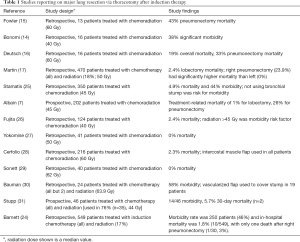
Full table
Subsequent larger retrospective studies have expanded on the perioperative risks of induction therapy. One of the largest published experience with thoracotomy after induction chemotherapy is a retrospective review of 470 patients (297 lobectomy, 97 pneumonectomy, 18 segmentectomy or wedge resection, 58 exploration only) treated at Memorial Sloan Kettering between 1993 and 1999, where 85 patients (18%) also received radiation (median dose 50 Gy, range 10 to 72 Gy) (17). Overall mortality was 3.8% (2.4% for lobectomy and 11.3% for pneumonectomy) and morbidity was 38.1%. All deaths after pneumonectomy occurred after a right-sided procedure, for which the mortality was 23.9%. A follow-up study from the same institution showed improved outcomes when they examined 549 patients who underwent surgery after induction chemotherapy in subsequent years [2000–2006], among whom 17% also had radiation (24). In this cohort, the morbidity rate was 46% and in-hospital mortality was 1.8%, with only one death after right pneumonectomy (3%).
Several other relatively large studies have reported results of patients who had been treated with both induction chemotherapy and radiation prior to lung resection. Stamatis et al. reported a hospital mortality of 4.9% and an overall morbidity of 44% for 350 patients treated with induction chemoradiation in a retrospective review of two phase II studies and one phase III study from 1991 to 2000 (25). Fujita et al. found that radiation dose greater than 45 Gy was a morbidity risk factor in another retrospective review of 124 patients who received chemoradiation between 1990 and 2003 (26). In this study, 90 patients had lobectomy and 25 patients had pneumonectomy with an overall 30 day mortality of 2.4%. Other relatively small retrospective studies have demonstrated similar surgical outcomes. Yokomise et al. reported on a single-surgeon series of 41 patients who were treated with platinum-based chemotherapy with concurrent radiation (50 Gy) followed by surgical resection (lobectomy or bilobectomy 28 patients, pneumonectomy 13 patients) with no mortalities (27).
Several studies have documented outcomes of patients who had received higher doses of radiation. Cerfolio et al. reported a mortality of 2.3% and overall morbidity of 37% (17% considered major) for 216 patients treated with induction chemotherapy and high dose radiation (median dose 60 Gy, range 60–72 Gy) followed by thoracotomy (lobectomy/bilobectomy 152, pneumonectomy 11, segmentectomy 14, wedge or no resection 35), where all patients had their bronchial stumps buttressed by intercostal muscle flaps (28). Sonett et al. reported 0% operative mortality for 40 patients (29 lobectomies and 11 pneumonectomies) with non-small cell lung cancer that had received neoadjuvant therapy that included thoracic radiation (mean 62 Gy, range 59.4 to 66.6 Gy) and concurrent platinum-based chemotherapy (29). Bauman et al. reported on 24 patients who were treated with definitive chemoradiation (median radiation dose 63.9 Gy with a range of 59.4 to 70.2 Gy, 2 of the patients received radiation alone) for locally advanced non-small cell lung cancer, and then subsequently underwent salvage lung resection for local recurrence (30). There was 1 perioperative death and an overall morbidity of 58%, with the most common complications being supraventricular tachycardia (29%), pneumonia (17%), vocal cord palsy (17%), tracheostomy (8%), and major vessel injury (8%). Yang et al. also reported on 31 patients that underwent lobectomy after curative-intent definitive radiotherapy (60 Gy), with or without chemotherapy with no perioperative deaths and 48% morbidity (12).
Results after lung resection via VATS after radiation
The use of VATS to achieve anatomic lung resections such as lobectomy was first reported in the 1990s (32). Both single-institution and multi-institution studies subsequently demonstrated VATS lobectomy to be safe and feasible, and then benefits over thoracotomy were shown in controlled studies (33-37). Advantages of VATS pulmonary resections include a lower incidence of postoperative complications, shorter length of chest tube duration and hospital stay, decreased post-operative pain, and preserved pulmonary function (34-36,38-42). A minimally invasive approach was shown to be particularly beneficial for higher risk patients such as older patients and patients with impaired pulmonary function (43-45). The widespread adoption of VATS lobectomy was somewhat slow, but 62% of lobectomies reported to the STS General Thoracic Database in the years 2012–2014 were done via VATS (1,34,35,38,39).
Initially, only patients with small, peripheral, early stage tumors were felt to be appropriate candidates for resection via VATS. Prior thoracic radiation and the use of induction therapy were relative contraindications (46). However, the use of VATS has now been reported in more advanced situations such as large and central tumors with clinically positive nodal disease as well as for achieving sleeve resections, chest wall resections, and pneumonectomy (47-51). The safety and feasibility of thoracoscopic lobectomy after induction therapy that includes radiation therapy has also been demonstrated in relatively small studies from generally high volume VATS institutions (12,52-57) (Table 2).
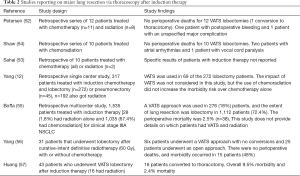
Full table
Performing VATS after radiation therapy
Operative planning
Although the studies discussed above has demonstrated the safety and feasibility of performing VATS after radiation therapy, it is important to note that all individual surgeons must carefully evaluate every situation if VATS is considered after radiation therapy. Surgeons must consider not only patient characteristics, but their own VATS experience, skill, and comfort level, as well as their institutional experience in terms of operative and perioperative support. Surgeons should probably not consider a VATS resection after radiation until they have attained an appropriate comfort level with less complex VATS anatomic resection procedures in general.
The process of avoiding complications when attempting a resection after radiation via VATS is generally no different than the process for any lung resection, and is dependent on appropriate preoperative workup and patient selection as well as very meticulous and careful intra-operative dissection and attention to detail (58,59). As with all most surgical procedures, the optimal strategy of managing complications of VATS pulmonary resections is to prevent their occurrence. Consideration of the radiographic appearance of the area of lung to be removed and the anticipated technical aspects in terms of hilar dissection is especially important when doing VATS after radiation. In these cases, focusing on hilar calcifications and adenopathy at the origin of the lobar bronchus that is to be divided as well as the number and locations of pulmonary artery and vein branches can allow surgeons to anticipate areas where dissection may be most difficult. The surgeon must always be ready and have the instruments needed to convert to thoracotomy if necessary.
Pre-operating staging and work-up for thoracoscopic resection should be the same in general as for thoracotomy, which includes checking pulmonary function tests (PFTs) with diffusion measurements (58-60). The only absolute contraindication to VATS resection from a patient’s clinical standpoint is the inability to tolerate single-lung ventilation, which involves very careful consideration of the patient’s contralateral lung status. Although VATS resections have been shown to be able to be accomplished in patients with lung function that have typically been thought to be too poor to undergo more conventional resection via thoracotomy, it’s imperative to consider that conversion to thoracotomy is possible for all patients for whom VATS resection is planned (61,62). Absolute tumor size criteria that would preclude VATS resections have not been defined, though large specimens may not be amenable to removal without rib spreading (60). In general, chest wall involvement requires thoracotomy for resection, though VATS can be used to perform the hilar portion of the surgery and allow placement of the incision better situated for the area of chest wall to be removed.
Pre-operative planning must involve not only the work-up described above, but also consideration of any intra-operative complications. The surgeon must anticipate and plan for potential complications such as massive hemorrhage and bronchial complications, as well as how to deal with unexpected equipment malfunctions. Additionally, the surgeon must at all times be sure to perform the same cancer operation with VATS as would be done with an open approach, utilizing individual vessel dissection and ligation with complete mediastinal lymph node dissection.
Conversion to thoracotomy
A very important consideration is always keeping in mind that conversion to thoracotomy is possible or even likely. The instruments needed for thoracotomy also must always be immediately available during thoracoscopic surgeries. Conversion to thoracotomy is a tool available to manage unexpected or difficult situations, and is not a complication in and of itself. Conversions may be due to difficulties with the procedure, including a narrow view angle, complicating conditions such as pleural adhesions, obscured tissue planes, or dense hilar lymphadenopathy, oncologic considerations such as the discovery of more extensive local disease than expected, and the surgeon’s discomfort with VATS visualization and instruments (60). Although unexpected conversion to thoracotomy during VATS does not necessarily compromise prognosis, the decision to convert must be made promptly to reduce operating time, blood loss, and other possible complications (63,64). In cases where dissection is particularly difficult, the surgeon should strongly consider conversion in an elective manner before something occurs that requires emergent conversion and possibly patient instability (65). Accordingly, when attempting a VATS procedure, access ports must be placed to facilitate immediate conversion to open thoracotomy and to support instrument manipulation and anatomic accessibility of the stapler to close vessels and the bronchus (60).
Conversion rates for thoracoscopic lobectomy to open thoracotomy have been reported to range from 2% to as high as 23%, with these higher rates generally observed in patients with more advanced NSCLC (35,39,47,66-71). Causes of conversion are generally classified into four categories: intraoperative complications, technical problems, anatomical problems, and oncological conditions (60). Reasons for conversions can include bleeding from hilar structures, failure to make progress, poor visualization, equipment malfunction, and oncological reasons such as centrally located tumors requiring vascular control or sleeve resection, unexpected T3–T4 tumors that invade the chest wall, diaphragm, or superior vena cava. Conversion may also be required due to abnormal/adherent hilar nodes secondary to a prior inflammatory process or metastatic disease, diffuse pleural adhesions, absent or thick fissure, tumor size precluding removal through the utility incision, invasion of hilar structures, or positive margins that require additional resection. Patients do not necessarily have higher morbidity or mortality directly as a result of conversion to open thoracotomy (60,63). However, patients who have a conversion have been shown to have higher blood loss and longer operative times (64,72). Therefore, with a focus on a safe and complete resection, conversion should be regarded as a means of completing resections in a traditional manner rather than as a surgical failure.
Anatomic considerations
Anatomic considerations that increase the difficulty of successfully performing VATS after radiation include obliteration of tissue planes, pleural adhesions, and the absence of fissures (60). Induction therapy, and radiation in particular, can distort normal anatomy due to perihilar fibrosis, leading to thicker and more adherent tissue planes around the hilar structures. Dissection in this situation may require sharp dissection, as blunt dissection or the use of cautery may not be feasible due to the obscured tissue planes. True pleural symphysis that requires conversion to thoracotomy may be unlikely for high volume VATS surgeons, but it may represent a contraindication to continuing via VATS in surgeons without extensive experience. Although creating space and entering the correct pleural plane can be difficult in the setting of dense adhesions, endoscopic adhesiolysis can often proceed quickly and safely using a combination of sharp and blunt dissection under videoscopic vision (60). VATS has the advantage over conventional thoracotomy in providing high resolution visualization of areas such as the apex and base of the hemithorax, which can be difficult to see even during thoracotomy when adhesions are present. In addition, successful VATS lobectomy can be accomplished in the setting of fused fissures, where the fused fissure is divided last, after dissection and division of the pulmonary vasculature and the bronchus (60).
One of the most dreaded complications for surgeons during anatomic lung resections is massive bleeding from pulmonary vessels (60). Dense adhesive disease that can occur after radiation often increases the risk of vascular injury during dissection, as described above. Dissection of vessels can generally be difficult in this situation, and risk of vessel injury and bleeding can be high even by thoracotomy. VATS pulmonary resections can be performed by capable VATS surgeons without an increased bleeding risk, probably at least partly facilitated by the visual magnification provided by the thoracoscope, but extreme care is required. As described above, sharp dissection may be necessary. Flexibility with the use of the thoracoscope can in some cases reduce the chance of vessel injury and significant bleeding. For example, movement of the thoracoscope from one port to another can improve or enhance visualization of the hilum (46). However, as described above, surgeons must carefully evaluate pre-operative imaging so they are aware of potentially dangerous or difficult areas, meticulously dissect planes in the hilum, and be ready to convert to thoracotomy at all times and preferably before a major vascular injury occurs when the dissection is difficult. In addition, surgeons may wish to obtain proximal control of the main pulmonary artery if the hilar dissection proves to be difficult.
Other methods to prevent complications
Bronchial stump reinforcement with muscle or a pleural flap may be considered after induction therapy to reduce the chance of fistula formation (73). Many surgeons routinely use flap coverage of the bronchial stump. Although a benefit has never been definitively proven, the routine use of bronchial stump flap coverage in several of the studies discussed above may have been at least partially responsible for the low observed morbidity and mortality rates with major lung resection after radiation therapy. Although harvest of flaps such as the intercostal muscle is typically done via thoracotomy, the harvesting of this muscle with a VATS approach has been described (74,75). Surgeons that plan for VATS lobectomy after radiation therapy, particularly if higher doses of radiation were used, should seek to develop the skill needed to harvest this flap. If harvesting the intercostal muscle is not feasible via VATS, other options include a pleural flap or a flap of pericardial fat to cover the bronchial stump.
Although reports disagree on the role of induction chemotherapy and radiation on the development of acute lung injury and adult respiratory distress syndrome after lung resection, there is some consensus on a strategy to reduce the risks of patients after preoperative therapy: minimization of intravenous fluids and both the fraction of inspired oxygen and airway pressures as much as possible after anesthesia induction (23,76-81). Although a surgeon may be too focused on the technical performance of the procedure to manage these things directly through the case, they should make a point of reviewing these perioperative management strategies with anesthesia prior to the case, as the anesthesiologists may not necessarily recognize the increased risks that accompany lung resection after induction therapy.
Conclusions
Almost all published studies that examine major lung resection after induction therapy that included radiation are retrospective in nature. These studies generally indicate that this treatment is associated with high potential risks, which can be reduced by careful patient selection, meticulous operative technique, and perioperative management (13). Bronchial stump coverage with a vascularized flap probably reduces perioperative morbidity, particularly when the dose of preoperative radiation is more than 45 Gy and when pneumonectomy has been performed. Although the use of thoracotomy and VATS after radiation therapy has never been prospectively directly compared in the literature, both approaches may be feasible depending on the specific circumstances. However, surgeons must carefully consider each individual clinical situation, including tumor location, as well as their own VATS experience before attempting a resection via VATS after radiation therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.03.08). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. MFB serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Aug 2016 to May 2019. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol 2005;23:3257-69. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1-6. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7-14. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Rusch VW, Albain KS, Crowley JJ, et al. Surgical resection of stage IIIA and stage IIIB non-small-cell lung cancer after concurrent induction chemoradiotherapy. A Southwest Oncology Group trial. J Thorac Cardiovasc Surg 1993;105:97-104. [PubMed]
- Veeramachaneni NK, Feins RH, Stephenson BJ, et al. Management of stage IIIA non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg 2012;94:922-6. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Stephens SJ, et al. Long-Term Outcomes of Lobectomy for Non-Small Cell Lung Cancer After Definitive Radiation Treatment. Ann Thorac Surg 2015;99:1914-20. [Crossref] [PubMed]
- Berry MF, D’Amico TA. VATS versus thoracotomy for major lung resection after induction therapy. In: Ferguson MK. editor. Difficult Decisions in Thoracic Surgery, 2nd edition. London: Springer, 2011:145-53.
- Bonomi P, Faber LP, Warren W, et al. Postoperative bronchopulmonary complications in stage III lung cancer patients treated with preoperative paclitaxel-containing chemotherapy and concurrent radiation. Semin Oncol 1997;24:S12-123-S12-129.
- Fowler WC, Langer CJ, Curran WJ, et al. Postoperative complications after combined neoadjuvant treatment of lung cancer. Ann Thorac Surg 1993;55:986-9. [Crossref] [PubMed]
- Deutsch M, Crawford J, Leopold K, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy with thoracotomy in the treatment of clinically staged IIIA non-small cell lung cancer. Cancer 1994;74:1243-52. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149-54. [Crossref] [PubMed]
- Siegenthaler MP, Pisters KM, Merriman KW, et al. Preoperative chemotherapy for lung cancer does not increase surgical morbidity. Ann Thorac Surg 2001;71:1105-11. [Crossref] [PubMed]
- Perrot E, Guibert B, Mulsant P, et al. Preoperative chemotherapy does not increase complications after nonsmall cell lung cancer resection. Ann Thorac Surg 2005;80:423-7. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg 2008;85:1857-65. [Crossref] [PubMed]
- Hampel M, Dally I, Walles T, et al. Impact of neo-adjuvant radiochemotherapy on bronchial tissue viability. Eur J Cardiothorac Surg 2010;37:461-6. [PubMed]
- Grichnik KP, D'Amico TA. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Semin Cardiothorac Vasc Anesth 2004;8:317-34. [Crossref] [PubMed]
- Barnett SA, Rusch VW, Zheng J, et al. Contemporary results of surgical resection of non-small cell lung cancer after induction therapy: a review of 549 consecutive cases. J Thorac Oncol 2011;6:1530-6. [Crossref] [PubMed]
- Stamatis G, Djuric D, Eberhardt W, et al. Postoperative morbidity and mortality after induction chemoradiotherapy for locally advanced lung cancer: an analysis of 350 operated patients. Eur J Cardiothorac Surg 2002;22:292-7. [Crossref] [PubMed]
- Fujita S, Katakami N, Takahashi Y, et al. Postoperative complications after induction chemoradiotherapy in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2006;29:896-901. [Crossref] [PubMed]
- Yokomise H, Gotoh M, Okamoto T, et al. Induction chemoradiotherapy (carboplatin-taxane and concurrent 50-Gy radiation) for bulky cN2, N3 non-small cell lung cancer. J Thorac Cardiovasc Surg 2007;133:1179-85. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Jones VL, et al. Pulmonary resection after concurrent chemotherapy and high dose (60Gy) radiation for non-small cell lung cancer is safe and may provide increased survival. Eur J Cardiothorac Surg 2009;35:718-23. [Crossref] [PubMed]
- Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg 2004;78:1200-5. [Crossref] [PubMed]
- Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg 2008;86:1632-8. [Crossref] [PubMed]
- Stupp R, Mayer M, Kann R, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009;10:785-93. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Video-assisted thoracoscopic major pulmonary resections: technical aspects, personal series of 259 patients, and review of the literature. Surg Endosc 2004;18:1551-8. [PubMed]
- Park BJ, Zhang H, Rusch VW, et al. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg 2007;133:775-9. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9. [Crossref] [PubMed]
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. [Crossref] [PubMed]
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Onaitis M, D’Amico TA. Lung Cancer: Minimally Invasive Approaches. In: Selke FW, del Nido PJ, Swanson SJ. editors. Sabiston and Spencer Surgery of the Chest, 7th edition. Philadelphia: Elsevier Saunders, 2005;1:277-84.
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Shiraishi T, Hiratsuka M, Yoshinaga Y, et al. Thoracoscopic lobectomy with systemic lymph node dissection for lymph node positive non-small cell lung cancer--is thoracoscopic lymph node dissection feasible? Thorac Cardiovasc Surg 2008;56:162-6. [Crossref] [PubMed]
- Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8. [Crossref] [PubMed]
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92. [Crossref] [PubMed]
- Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-709. [Crossref] [PubMed]
- Boffa D, Fernandez FG, Kim S, et al. Surgically Managed Clinical Stage IIIA-Clinical N2 Lung Cancer in The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:395-403. [Crossref] [PubMed]
- Yang CJ, Mayne NR, Wang H, et al. Outcomes of major lung resection after induction therapy for non-small cell lung cancer in elderly patients. Ann Thorac Surg 2016;102:962-70. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Berry MF, D’Amico TA. Complications of Pulmonary Resection: Lobectomy & Pneumonectomy. In: Little AG, Merrill WH. editors. Complications in Cardiothoracic Surgery: Avoidance and Treatment, Second Edition. Wiley-Blackwell, 2010;158-81.
- Berry MF, D'Amico TA. Complications of thoracoscopic pulmonary resection. Semin Thorac Cardiovasc Surg 2007;19:350-4. [Crossref] [PubMed]
- Hanna JM, Berry MF, D’Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-S189. [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: A case control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg 2008;86:235-9. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Winter H, Meimarakis G, Pirker M, et al. Predictors of general complications after video-assisted thoracoscopic surgical procedures. Surg Endosc 2008;22:640-5. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Gharagozloo F, Tempesta B, Margolis M, et al. Video-assisted thoracic surgery lobectomy for stage I lung cancer. Ann Thorac Surg 2003;76:1009-14; discussion 1014-5. [Crossref] [PubMed]
- Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2001;72:879-84. [Crossref] [PubMed]
- Krasna MJ, Deshmukh S, McLaughlin JS. Complications of thoracoscopy. Ann Thorac Surg 1996;61:1066-9. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519-23. [Crossref] [PubMed]
- Serna-Gallegos DR, McKenna RJ Jr. Video-Assisted Intercostal Muscle Flaps for Bronchial Stump Coverage. Ann Thorac Surg 2017;103:e215-e217. [Crossref] [PubMed]
- Sagawa M, Sugita M, Takeda Y, et al. Video-assisted bronchial stump reinforcement with an intercostal muscle flap. Ann Thorac Surg 2004;78:2165-6. [Crossref] [PubMed]
- Busch E, Verazin G, Antkowiak JG, et al. Pulmonary complications in patients undergoing thoracotomy for lung carcinoma. Chest 1994;105:760-6. [Crossref] [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [Crossref] [PubMed]
- Dulu A, Pastores SM, Park B, et al. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006;130:73-8. [Crossref] [PubMed]
- Ruffini E, Parola A, Papalia E, et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001;20:30-6. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91. [Crossref] [PubMed]
- Lee HS, Lee JM, Kim MS, et al. Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg 2005;79:405-10. [Crossref] [PubMed]
Cite this article as: Berry MF. Advanced VATS resections: radiation therapy and VATS. Video-assist Thorac Surg 2018;3:18.


