
Stereotactic body radiotherapy for early-stage non-small cell lung cancer: a surgical perspective on the American Society for Radiation Oncology Guidelines
Stereotactic body radiation therapy (SBRT) has emerged as a safe and effective treatment modality for selected patients with non-small cell lung cancer (NSCLC) (1). Compared with other radiation techniques, SBRT is delivered with fewer fractions of higher-dose radiation that are directed more precisely at located targets, providing a higher biologically effective dose while minimizing toxicity to the surrounding normal tissue. Since the introduction of SBRT to clinical practice more than a decade ago, there has been growing interest in its role in the treatment of NSCLC, as demonstrated by the increasing number of publications on SBRT in the literature (Figure 1) (2-4). Historically, clinical data on SBRT were from patients with significant comorbidities who were not suitable surgical candidates (4). However, recent studies have expanded the patient cohort to high-risk surgical candidates who can tolerate either surgical resection or SBRT (5,6). The authors of a retrospective subgroup analysis of two prematurely terminated randomized controlled trials commented that SBRT may even be superior to lobectomy, in terms of overall survival, for early-stage operable candidates—a claim that is challenged by most surgeons (7,8).
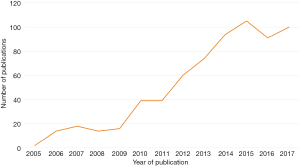
To clarify the existing clinical evidence on SBRT for the treatment of NSCLC, the American Society for Radiation Oncology (ASTRO) recently produced a set of evidence-based guidelines, which have been endorsed by the American Society of Clinical Oncology (ASCO) and published in the Journal of Clinical Oncology (9). These guidelines were produced by a task force approved by ASTRO and are based on a literature search from 1995 to April 2017. The guidelines specifically address a number of key clinical questions, separated primarily by the operability of the patient cohort. For operable surgical candidates, when is SBRT appropriate for patients with T1-2, N0 NSCLC? For nonoperable surgical candidates, when is SBRT appropriate for patients who have tumors of a particular size, location, or with synchronous or multifocal presentation and who have had previous surgery or radiotherapy?
From a surgical perspective, the most important clinical question addressed by the ASTRO guidelines relates to the patient selection process for operable patients with T1-2, N0 NSCLC. The guidelines make three key recommendations for this patient cohort.
- Patients with stage I NSCLC should be assessed by a thoracic surgeon for operability.
- For patients with standard operative risk and stage I NSCLC, SBRT is not recommended outside of a clinical trial.
- For patients with high operative risk and stage I NSCLC, discussions about SBRT should be encouraged, and SBRT should be considered an alternative treatment to surgery.
Further qualifying statements by ASCO point out the lack of randomized data comparing SBRT to lobectomy or sublobar resection, the limited long-term data on SBRT beyond 5 years, and the difficulty of assessing and defining operative risks.
Several key challenges remain to identify the appropriate role of SBRT for operable patients. First, there is a paucity of robust long-term data on locoregional control for SBRT beyond 5 years. This becomes more important as life expectancy increases in an aging population and as SBRT is offered to younger patients. A patient should be fully informed of the lack of long-term data on SBRT, especially compared with established surgical outcomes from historical data. Future studies should strive to obtain long-term clinical data with clearly defined clinical and radiographic follow-up regimens to assess for locoregional control. Second, there is a heterogeneous requirement for histologic confirmation before treatment with SBRT, especially in Europe, where up to 60% of treated patients have no recorded histologic diagnosis (10). This likely has an influence on overall, disease-free, and cancer-specific survival, to an unknown extent. To address this issue, the ASTRO guidelines strongly recommend that clinicians obtain a biopsy before treatment with SBRT to confirm that a nodule is malignant in nature. However, they also provide a moderate recommendation to allow SBRT in patients who refuse or fail biopsy or are considered to have prohibitive risks for biopsy after discussion within a multidisciplinary team. Another consideration when comparing SBRT to surgery is that much of the surgical data originated from historical records before the introduction of minimally invasive surgery (11). Abundant evidence suggests VATS produces superior perioperative outcomes and similar if not superior long-term outcomes compared with open thoracotomy (12,13). Future comparative studies of SBRT versus surgery need to include higher proportions of patients who undergo VATS lobectomy or segmentectomy and mediastinal lymph node dissection/sampling, which is the standard treatment for operable patients with early-stage NSCLC (14). Finally, it should be acknowledged that there are limited data on quality of life and cost-effectiveness outcomes between SBRT and surgery.
In summary, ASTRO and ASCO should be commended for producing a set of guidelines in a timely manner to help guide physicians and surgeons in their clinical practice and to update the available clinical data on SBRT. We agree that it is absolutely necessary for a thoracic surgeon to assess a patient with resectable NSCLC before consideration of SBRT. We acknowledge that there is a significant and perhaps growing role for SBRT for high-risk patients who are considered inoperable or very high risk for perioperative adverse outcomes. These patients should, in our opinion, have a pretreatment histologic diagnosis and have long-term clinical and radiographic follow-up to assess for locoregional control after SBRT. Although the ASCO Expert Panel considers the recommendations from these guidelines to be clear, thorough, and based on the most relevant clinical evidence, they also note a lack of strong evidence for many of the recommendations in the guidelines. Therefore, they emphasize the importance of shared decision making between surgeons, physicians, patients, and their families. With maturing long-term data and the initiation of more carefully designed prospective studies, we hope that the role of SBRT will become better defined in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Monisha Sudarshan (Mayo Clinic Rochester, Minnesota, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.03.01). DRJ serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Aug 2016 to May 2019. DRJ serves as a senior medical advisor for Diffusion Pharmaceuticals and a consultant for Merck and AstraZeneca. CC has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Song DY, Benedict SH, Cardinale RM, et al. Stereotactic body radiation therapy of lung tumors: preliminary experience using normal tissue complication probability-based dose limits. Am J Clin Oncol 2005;28:591-6. [Crossref] [PubMed]
- Navarro-Martin A, Aso S, Cacicedo J, et al. Phase II Trial of SBRT for Stage I NSCLC: Survival, Local Control, and Lung Function at 36 Months. J Thorac Oncol 2016;11:1101-11. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Cao C, D'Amico T, Demmy T, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e370-1. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Mokhles S, Nuyttens JJ, Maat AP, et al. Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol 2015;22:316-23. [Crossref] [PubMed]
- Parashar B, Port J, Arora S, et al. Analysis of stereotactic radiation vs. wedge resection vs. wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy 2015;14:648-54. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
Cite this article as: Cao C, Jones DR. Stereotactic body radiotherapy for early-stage non-small cell lung cancer: a surgical perspective on the American Society for Radiation Oncology Guidelines. Video-assist Thorac Surg 2018;3:11.


