
Video-assisted thoracoscopic surgery (VATS) for central airway tumors: VATS carinal resection and reconstruction
Introduction
For nearly seven decades, carinal surgery has remained challenging for thoracic surgeons due to technical difficulties, including the administration of anesthesia during the reconstruction and the anatomical technique of carinal reconstruction (1-4). Even in appropriately selected patients treated at centers with experience, carinal surgery in open thoracotomy is associated with major issues and is accompanied by high morbidity and mortality rates of 39.0–50.0% and 7.1–22.2%, respectively (5-7).
Since the introduction of video-assisted thoracoscopic surgery (VATS) to major thoracic operations, such as pulmonary lobectomy, approximately a quarter-century ago (8,9), many challenges associated with the use of VATS to treat locally advanced lung cancer have been described (10-17). However, despite widespread doubt that a VATS approach would be suitable in the setting of extremely difficult carinal surgery, a successful case of VATS carinal resection and reconstruction was first reported in 2013 (18). While surgeons encountered several difficulties during carinal reconstruction in that case, further improvements in surgical skills and the technological advancement of thoracoscopic instruments are expected to allow more surgeons to perform VATS carinal surgery.
This review article focuses attention on the feasibility of airway management and technical strategies for complex anastomosis during carinal reconstruction under a VATS approach, which includes rapidly evolving surgical technologies. Between April 1992 and March 2017, literature published worldwide and written in English were retrieved from a listing of bibliographic references obtained from the National Institutes of Health (https://www.ncbi.nlm.nih.gov/pubmed) using the specific keywords “VATS”, “thoracoscopic”, “carinal”, and “reconstruction”. The retrieved studies consisted entirely of case reports and small case series, probably due to the rare nature of the relevant diseases. There were eight reports of VATS carinal resection and reconstruction and four reports of VATS pneumonectomy with carinal reconstruction, and their results regarding ventilation strategy, surgical techniques, and postoperative complication are listed in Tables 1 and 2, respectively. The Tables may include overlapping cases because there were several reports including the same institutions and coauthors. Technical issues specific to the VATS approach including airway management, port strategy, reduction of anastomotic tension, extent of resection, modes of reconstruction, suturing techniques, types of suture thread, and prophylactic wrapping, are also discussed in this review.
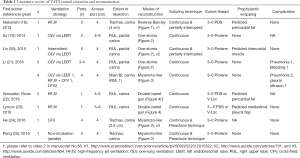
Full table
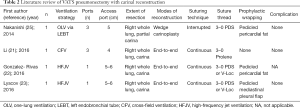
Full table
Airway management
Given that almost all of the reports described VATS procedures for the carina through the right thoracic cavity, one-lung ventilation (OLV) via the left endobronchial tube was used for airway management without tumor involvement of the left main bronchus before carinal surgery (18-24,26). Four reports showed that the OLV via the left endobronchial tube was sequentially used during partial resection and reconstruction of the carina (Tables 1 and 2) (19-21,26). Lin et al. reported that the intermittent use of OLV via the left endobronchial tube played an important role in airway management during reconstruction of the partially removed carina (20). Although “one-stoma type” included among the reconstructive techniques for correction of the partially removed carina helped surgeons avoid removing the left endobronchial tube, intermittent OLV may be performed with the cooperation of surgeons and anesthesiologists only when it is hard to stitch one-stoma reconstruction.
When the left main bronchus was transected in the first stage of sleeve resection of the carina, the left main bronchus routinely was intubated using a surgical field tube (approximately 6.0 mm in diameter, in which a sterile spiral was embedded) for cross-field ventilation. Thereafter, high-frequency jet ventilation (HFJV) through a narrow catheter was performed during sleeve resection and reconstruction of the carina in four reports (Tables 1 and 2) (18,21-23). HFJV, which Sanders first reported as a method of ventilation via a bronchoscope (27), was widely applied for airway surgery because it provided a better view and equivalent ventilation compared with cross-field ventilation (28,29). However, care should be taken when establishing HFJV because barotrauma, such as pneumothorax, mucosal trauma caused by dry gas, and hypoxia and/or hypercapnea due to impaired ventilation are associated with adverse effects of HFJV (30). In addition, whipping of the catheter tip due to delivery of the gas jet can cause mechanical injury to the bronchial mucosa when the catheter is made of soft material.
In the setting of HFJV, the impairment of carbon dioxide elimination is associated with an increased respiratory rate, decreased driving pressure, and reduced inspiratory time (31). Typical settings for HFJV are as follows: a respiratory rate, 150 cycle/minute (2.5 Hz); driving pressure, 2.0 kg/cm2; inspiratory time, 50%; and inspired oxygen fraction (FiO2), 1.0 (30). The authors complied with this setting, but halving the driving pressure and inspiratory time was sufficient in a previous patient (18). In the authors’ experience, the configuration of the tip of a narrow catheter also was associated with a successful operation. The authors selected a blocker tube with a deflated balloon for the following three reasons: First, the blocker tube helped achieve OLV without causing any injury to the tumor. Second, the deflated balloon prevented the bronchial mucosa from causing mechanical injury because the deflated balloon kept the tip of the tube nearly immobilized and served as shock-absorbing material during HFJV in the bronchus (18). Third, hypothetically speaking, balloon inflation protects the left main bronchus from the aspiration of blood, even if sudden massive bleeding occurs. HFJV via the blocker tube with a deflated balloon can therefore resolve concerns about tube management interfering with the surgical field and ventilation causing complications such as hypoxia and/or hypercapnea.
Patients with combined abnormalities of the lung function, such as chronic obstructive pulmonary disease, and male patients with an elevated body weight may be contraindicated for HFJV, as impairment of carbon dioxide elimination is often found in such patients (31,32). Porhanov et al. claimed that the use of HFJV may be a risk factor for the development of acute respiratory distress syndrome (ARDS) (33), although many investigators have reported that the physiology of HFJV seems to make it an ideal modality for patients with acute lung injury or ARDS (30,34,35).
Two reports described cross-field ventilation using a surgical field spiral tube being sequentially performed during carinal reconstruction (Tables 1 and 2) (21,24). He et al. reported that cross-field ventilation is a traditional and trustworthy airway management method, which may increase the risk of ARDS, as reported by Porhanov et al. (24,33). However, a surgical field tube prevents surgeons from stitching the left wall, farthest from the operating surgeon, when performing anastomosis between the trachea and the left main bronchus. When the anastomotic portion undergoes an increase in tension, it is too difficult to ligate or to stitch. A parachute suture technique may be a good way to anastomose when surgeons have difficulty visualizing the far side of the tube (24).
When delivering anesthesia during carinal surgery, there are only two methods for avoiding the need for troublesome airway management: lung assist using extracorporeal circulation and innovative non-intubated anesthesia. Extracorporeal lung assist is an ideal way to reconstruct the carina when airway management is difficult because of tumor involvement of the carina. Woods et al. reported the first successful case of simple reconstruction of the carina using the extracorporeal circulation (36). However, several investigators have reported unsuccessful cases complicated with hemorrhaging due to the use of even low-dose heparin (4,37). The indications for the extracorporeal lung assist may be limited to patients who cannot receive usual airway management by an anesthesiologist due to tumor involvement of the carina.
Regarding non-intubated anesthesia, Mukaida et al. first reported thoracoscopic wedge resection for pneumothorax under local and epidural anesthesia in 1998 (38). Thereafter, Pompeo et al. reported that the non-intubated thoracoscopic approach was superior to the conventional thoracoscopic approach under general anesthesia for the treatment of benign diseases (39-43) as well as for the immune systems of patients (44,45). Furthermore, Chen et al. reported that non-intubated thoracoscopic lobectomy was safe and technically feasible in select patients with early-stage non-small cell lung cancer (46-48). Finally, Peng et al. reported highly successful results in performing VATS carinal resection and reconstruction under non-intubated anesthesia for one carefully selected patient (Table 1) (25).
We hesitated to perform non-intubated VATS major surgery because a randomized control study reported that the non-intubated anesthesia group tended to have greater intraoperative blood loss during major surgery than the conventional one-lung anesthesia group (49). If bleeding suddenly occurs, it can be extremely difficult to control in a limited working space due to spontaneous breathing of the affected lung (50). We were also concerned about avoiding aspiration of the blood and secretion into the left main bronchus and administering local anesthesia to the left main bronchus. Such an innovative surgery might have been achieved due to the fact that Peng’s center had a great deal of experience in performing non-intubated surgeries.
Port strategy
Six of seven investigators performed VATS carinal resection and reconstruction using 3 to 5 ports, including an access port 4 to 6 cm in length, while Gonzalez-Rivas et al. reported an innovative uniportal strategy with a port 4 to 6 cm in length that led to the first successful VATS carinoplasty (22,23) (Tables 1 and 2). Despite the conventional VATS procedures using multiple ports, thus complicating the already complex carinal surgery because of two-dimensional imaging and restricted handling of surgical instruments compared with open thoracotomy, Gonzales-Rivas et al. successfully performed VATS carinoplasty using as few ports as possible (22).
Over the past two years, Gonzales-Rivas et al. have reported a successful uniportal VATS series including lobectomy (51), sleeve lobectomy (52,53), lobectomy with pulmonary arterioplasty (54), double-sleeve lobectomy (55), and pneumonectomy (56). These authors have demonstrated exceptional ability and improved their skills in a stepwise manner, which led to their successful performance of uniportal VATS carinoplasty. They showed that complex uniportal VATS procedures should be performed either by or with skilled and experienced VATS surgeons to ensure safety and avoid complications (57). At present, uniportal VATS carinoplasty seems to be the hardest surgery because of the excessively restricted handling of surgical instruments and therefore requires several super-minimally invasive surgical techniques. These authors developed ideal strategies to ensure their surgery’s success. We therefore believe that their strategies, including airway management using HFJV, double-barrel reconstruction by creating neocarina, and a continuous suturing technique using absorbable barbed sutures, may facilitate multiport VATS procedures for carinal surgery. Each of the above-mentioned strategies is discussed in detail below.
Reduction of anastomotic tension
All of the investigators reported that the distal trachea and both main bronchi were fully released, with minimal harm to the vascular supplies to the airway, in order to reduce any unnecessary tensile force during anastomosis after dividing the pulmonary ligament (18-26). Extended hilar mobilization including release of the pericardium around the pulmonary vein may be beneficial in some cases with heightened tension between the trachea and bronchus (4). There have been no reports on specific techniques for the reduction of anastomotic tension in the VATS carinal reconstruction except for our report. In our case, a technically demanding type of reconstruction was required to overcome the increased anastomotic tension because removal of the 4-cm-long airway, including the trachea and carina, was required. Therefore, we employed an innovative traction technique that included the use of a traction device with an endoscopic needle-like hook to reduce anastomotic tension (58). This technique is helpful for reconstruction of the long-segment airway including the carina.
Extent of resection and modes of reconstruction
Modes of carinal reconstruction are usually dependent on the extent of resection of the carina to the trachea. A variety of techniques for reconstruction of the carina have been published (4), but Table 1 shows only four types of carinal reconstruction feasible under VATS. It seems logical that there are fewer feasible modes of carinal reconstruction by VATS than by open thoracotomy, as VATS procedures always require deflation of the affected lung to secure a working space, and handling of surgical instruments is restricted during VATS procedures. Specifically, anastomosis between the right main bronchus and trachea followed by implantation of the left main bronchus into the bronchus intermedius, which Barclay et al. performed after removal of extensive trachea and carina (59), seems almost impossible to perform using VATS procedures because of the extreme difficulty associated with airway management to secure a working space. To perform such reconstruction successfully, implantation of the left main bronchus into the bronchus intermedius must be preceded during HFJV or cross-field ventilation of the left lung. HFJV is then required to introduce the left main bronchus via the bronchus intermedius. The “reverse” Barclay’s procedure, consisting of anastomosis between the left main bronchus and trachea followed by implantation of the right bronchus into the left main bronchus, can be performed with easier management of the airway and therefore seems possible even by VATS procedures (4,18) (Figure 1).
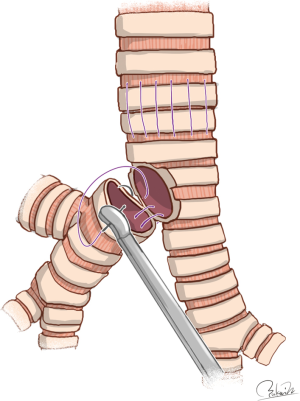
Naturally, anastomotic tension between the trachea and left main bronchus must be reduced to allowable levels. Dartevelle and Macchiarini showed that the safe limit of carinal resection is approximately 4 cm between the lower trachea and contralateral main bronchus (60). Porhanov et al. also reported carinal resection of 2–4 cm to be safe because there was a significant difference in the mean length of carinal resection by survival (5.3 cm in non-survivors versus 3.4 cm in survivors) (33). Because the extent of resection between the trachea and carina was 4 cm in length in our previous case, we employed the “reverse” Barclay’s procedure for VATS carinal reconstruction after sufficient mobilization (18).
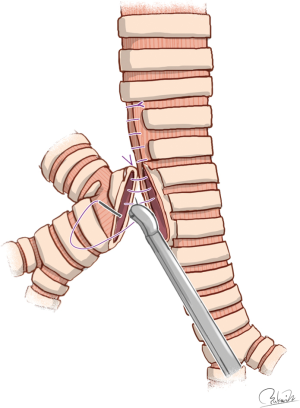
The mode of carinal reconstruction was one-stoma type when the carina was partially removed (61). This reconstruction mode seems easier than other reconstructions after sleeve resection of the carina because of single anastomosis and flat airway management. However, it is difficult to match the aperture of the trachea with the caliber of the bronchus intermedius and to perform end-to-side anastomosis using VATS procedures. All three reports found that surgeons had to get creative with matching the tracheal aperture with the caliber of the bronchus intermedius. Specifically, the side of the lower segment of the trachea was first sutured to narrow the rim of the tracheal aperture to better match the caliber of the bronchus intermedius (19-21) (Figure 2). In end-to-side anastomosis which is technically demanding, continuous suturing with a parachute technique may be used with the use of a nerve hook to prevent thread loosening (20).
Sleeve resection of the carina without pneumonectomy requires double anastomoses. The following three methods have been described: above-mentioned “reverse” Barclay’s reconstruction, carinal reconstruction performed in a manner analogous to Miyamoto et al., and a “double-barrel gun” type reconstruction (Table 1). Miyamoto’s technique includes the first anastomosis between two-thirds of the tracheal end and the left main bronchus and the second anastomosis between one-third of the tracheal end, which is trimmed to create an oval-shape orifice, and the right bronchus in an end-to-side fashion (7). He et al. reported their techniques, which included the first anastomosis between the tracheal end and three-quarters of the left main bronchus and the second anastomosis between the right bronchus and the aperture, which was made after the completion of the first anastomosis (24) (Figure 3). These surgeries require technical skills and innovative sense because double anastomoses are instrumental in the success or failure of carinal reconstruction.
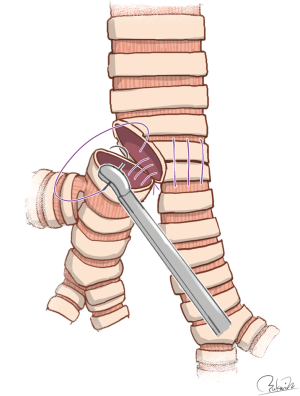
Regarding double-barrel gun type carinal reconstruction, Hardin and Fitzpatrick first reported successful results (62). Gonzalez-Rivas et al. reported an innovative technique for uniportal VATS carinal reconstruction using easy-to-follow illustrations. Their technique included the first anastomosis of the left-sided wall between the trachea and left main bronchus and the second anastomosis of the remnant wall between the trachea and bronchi after creation of neocarina resembling a double-barrel gun (22) (Figure 4). Even though the surgical conditions were severe, their technique appears to be simple and feasible. Although they performed airway management using HFJV, their procedures may allow for the use of cross-field ventilation.
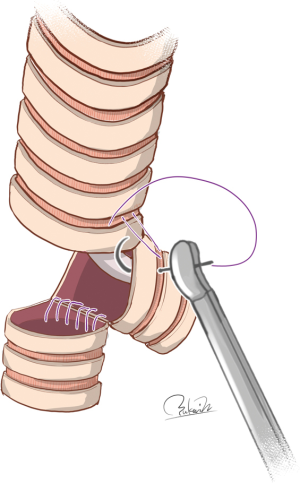
However, Grillo previously explained that even double-barrel gun type reconstruction has weaknesses (4). Briefly, this technique is applicable only in cases of very small tumors requiring limited resection of the carina, probably due to two directional tensile forces exerted on the neocarina. Specifically, anastomosis between the trachea and left main bronchus is accomplished by devolvement of the trachea down to the neocarina because the left main bronchus has very little mobility, even if blunt dissection is carried down its anterior surface (4). Because the other tensile force exerted on the neocarina is determined by the defect of the right bronchus and right hilar mobilization, a larger defect of the right bronchus and/or less mobilization may lead to anastomotic failure of the neocarina. In contrast, Gonzalez-Rivas et al. successfully performed double-barrel gun type reconstruction in two patients who required additional right upper lobectomy (22). A small extent of the resection between the lower trachea and left main bronchus may permit double-barrel gun type reconstruction because anastomotic tension between the lower trachea and right bronchus, which occurred in association with extensive removal of right bronchus, is largely offset by extensive right hilar mobilization. Further studies may be needed in order to establish the feasibility of double-barrel gun type reconstruction after extensive resection of the airway including the carina.
The modes of carinal reconstruction for VATS pneumonectomy with wedge and sleeve resection of the carina include wedge carinoplasty and end-to-end anastomosis between the trachea and left main bronchus, respectively (Table 2). Since Mathey et al. first reported successful techniques and results, both modes of reconstruction are the most common carinal procedures (63). Although airway reconstruction after sleeve pneumonectomy seems easier than that after sleeve resection of the carina when pneumonectomy can be avoided because of single anastomosis and flat airway management, extreme care should be taken concerning the anastomotic tension between the lower trachea and left main bronchus, as we described previously.
Suturing technique
In carinal reconstruction under open thoracotomy, many surgeons recommend interrupted sutures in order to better match the caliber of the airway anastomotic portion, although continuous sutures have been occasionally used only on the deepest aspect of the airway with respect to the surgeon in end-to-end anastomosis (4-7). However, Kutlu and Goldstraw showed that continuous sutures were comparable to interrupted sutures in the anastomotic healing of the airway and recommended the use of continuous sutures because of the speed and technically ease of the procedure (64).
In this series of VATS carinal reconstruction, the use of continuous sutures increased over time (Tables 1 and 2). The main reasons for the trend toward continuous sutures among VATS surgeons include a decreased operative time caused by the reduced number of ligations, the prevention of any tangling of the sutures, and easier anastomoses. Continuous sutures, which are reliable in the tensile portion (65), have been experimentally certified as reliable for use in carinal reconstruction in animals (66,67).
However, continuous sutures occasionally prevent the anastomotic portion from being aligned in an end-to-end fashion, causing the anastomosis to become partially telescoping. This is not a cause for concern because a partially telescoping anastomosis maintains continuity of the airway. We believe that continuous sutures using three to four threads rather than a single thread will lead to a good outcome by matching the caliber of the tracheal end with that of the bronchus. In a similar concept, Gonzalez-Rivas et al. showed that continuous sutures performed in two steps for the posterior and the anterior bronchial wall resulted in less suture tangling and a quicker performance than interrupted sutures (57).
A parachute technique was used in a video included with two reports (24,25). This technique led to a speedy surgery, the prevention of tangling of the sutures, and easier stitching with end-to-side anastomosis and anastomosis of the far side of the tube. We believe that the needle and thread should not be pulled out from the wound at every stitch because tangling often occurs.
Types of suture thread
Few studies have examined the effect of the suture material used for airway reconstruction on wound healing (68-72). Shaw and Luke reported that polyglycolic acid as absorbable sutures (n=45) was associated with a lower rate of anastomotic complications than non-absorbable sutures, including cotton and synthetic materials (n=55), in 100 consecutive patients who underwent sleeve lobectomy (68). Grillo found absorbable sutures to be advantageous for tracheal reconstruction because of the decreased appearance of granulation tissue at the anastomosis (69). In an experimental study using rabbits, McKeown et al. showed that the use of polydioxanone (PDS; Ethicon, Inc, Somerville, NJ, USA) as absorbable sutures was associated with a lower stenotic ratio at tracheal anastomosis than the use of polypropylene (Prolene; Ethicon, Inc) as non-absorbable sutures (70). In contrast to the results of the above studies, Chinese investigators showed successful VATS carinal reconstruction using Prolene sutures (19-21,24,25) (Tables 1 and 2). Both PDS and Prolene sutures are synthetic monofilament sutures causing minimal tissue reaction, although their materials differ with regard to the absorption into the body. Therefore, the effect on the airway anastomosis of both sutures may be minimal and pose little risk to the patency of the airway. However, this issue may never be resolved, as new suture materials are continually being developed.
Two reports described successful airway reconstruction using a novel absorbable barbed suture device (V-LocTM 180, Covidien AG, New Heaven, CT, USA) that facilitates secure wound closure without knot-tying and is indicated for soft tissue approximation, where the use of absorbable sutures is appropriate (Tables 1 and 2). Bush et al. reported that tracheal anastomosis with continuous 3–0 V-Loc sutures was a feasible alternative to conventional closure with interrupted 3–0 absorbable, synthetic, and braided sutures due to their comparable tensile strength in a study of cadaveric human trachea (71). Nakagawa et al. showed that bronchial anastomosis using continuous 3–0 to 4–0 V-Loc sutures was feasible and safe in four patients undergoing sleeve lobectomy (72). Gonzalez-Rivas et al. performed successful surgeries based on the two above-described studies (22,23). While some concerns remain regarding whether or not the V-LocTM 180 sutures can maintain their tensile strength in long-segment airway reconstruction, if such concerns are eliminated, a novel endoscopic suturing device (Endo StitchTM, Covidien AG) with absorbable barbed sutures (V-LocTM 180, Covidien AG) may facilitate VATS airway reconstruction in the near future.
Prophylactic wrapping
Grillo’s report describing a patient’s death due to massive bleeding on the ninth postoperative day was particularly impressive (4). Although Grillo wrapped the anastomoses with either a pedicled pleural flap or an intercostal muscle pedicled flap in all patients, the patient in question had no tissues interposed between the anastomosis and the pulmonary artery. It is therefore highly possible that a fatal complication such as bronchovascular fistula occurred. We have since performed prophylactic bronchial coverage using a pedicled pericardial fat pad flap in order to prevent complications attributable to the techniques used for VATS bronchoplasty, including carinal reconstruction (15,18,26,58). Matsuoka et al. reported that even a free pericardial fat pad might contribute to good wound healing of the bronchial stump for at least five months after surgery (73). Although Tables 1 and 2 shows prophylactic coverage using a pedicled pericardial fat pad flap (18,22), a pedicled intercostal muscle flap (20), and a mediastinal pleural flap (23), there are several investigators who did not use prophylactic coverage. Prophylactic bronchial coverage may be recommended for patients undergoing VATS carinal reconstruction because several studies have reported that prophylactic bronchial coverage decreases the incidence of bronchial fistula (74-76).
Surgical outcome
Tables 1 and 2 show neither fatal complications nor obvious anastomotic complications. A satisfactory short-term outcome was obtained despite few reports with a median duration of follow-up exceeding 12 months. In our cases, one patient with pathologic stage IIIA (T2aN2M0) who required VATS pneumonectomy with wedge carinoplasty following induction chemotherapy is still alive without any signs of recurrence at 82 months after surgery. The other patient with pathologic stage IIIA (T4N0M0) who required VATS sleeve carinal resection and reconstruction is also alive without any signs of recurrence at 72 months after surgery. Both presented with no signs of stenosis of the reconstructed carina during follow-up. Reports of the long-term outcome of the VATS carinal surgeries listed in this review article are expected to be forthcoming.
Indication for VATS carinal resection and reconstruction
When the second diameter of the lesion is larger than the length of the access incision, which was around 5 cm in the retrieved reports, the lesion is understandably difficult to extract through the access incision without significant rib spreading. Regarding invasion to the surrounding organs, a few investigators have reported that partial removal of the superior vena cava (SVC) with lobectomy is feasible in patients with lung cancer (26,77). However, there are no reports of VATS treatment for invasion of the esophagus, aorta, or extensive SVC. From our successful experiences (18,26) and a report on association between the extent of resection and the prognosis (33), the safe limit of carinal resection is approximately 4 cm between the lower trachea and contralateral main bronchus, although the extent of resection varies among individuals.
A three-dimensional thoracoscope and robotic arm may reduce the vulnerability associated with such problems as the availability of only two-dimensional images and limited manipulation of the needle holder, respectively (78,79). Robotic surgery provides a three-dimensional view and easy manipulation of the needle holder, thereby allowing the surgeon to perform high-precision sutures, as in open thoracotomy (80,81). Although the indications for VATS carinal resection and reconstruction with or without anatomic pulmonary resection may be expanding with improvements in surgical skills and the technological advancement of thoracoscopic instruments, we believe that the current indication includes the second diameter of the lesion ≤5 cm, minimal invasion to the surrounding organs, such as low-grade invasion of the SVC, and extent of resection ≤4 cm between the lower trachea and contralateral main bronchus (Table 3).

Full table
Conclusions
VATS carinal resection and reconstruction remain challenging due to the limited modes of carinal reconstruction as well as problems specific to the VATS approach, such as airway management, reduction of anastomotic tension, and suturing techniques. However, VATS carinal surgery with or without anatomic pulmonary resection may be made feasible by adopting several approaches, including HFJV using a blocker tube, an effective traction technique using endoscopic devices, and continuous sutures, and should be performed either by or with skilled and experienced VATS surgeons. In addition, non-intubated anesthesia and a uniportal strategy for VATS carinal surgery may be feasible at centers with a great deal of experience, although such techniques seem particularly challenging. The current indications for VATS carinal surgery are quite narrow and include the second diameter of the lesion ≤5 cm, minimal invasion to the surrounding organ, and extent of resection ≤4 cm between the lower trachea and contralateral main bronchus.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.01.03). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. RN serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Aug 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Belsey R. Stainless steel wire suture technique in thoracic surgery. Thorax 1946;1:39-47. [Crossref] [PubMed]
- Abbott OA. Experiences with the surgical resection of the human carina, tracheal wall, and contralateral bronchial wall in cases of right total pneumonectomy. J Thorac Surg 1950;19:906-22. [PubMed]
- Friedmann JB, Emma E. A consideration of anesthesia during carinal resection. Anesthesiology 1951;12:740-4. [Crossref] [PubMed]
- Grillo HC. Carinal reconstruction. Ann Thorac Surg 1982;34:356-73. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52. [Crossref] [PubMed]
- de Perrot M, Fadel E, Mercier O, et al. Long-term results after carinal resection for carcinoma: Does the benefit warrant the risk? J Thorac Cardiovasc Surg 2006;131:81-9. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Surgical results of carinal reconstruction: An alternative technique for tumors involving the tracheal carina. Ann Thorac Surg 2007;84:216-20. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85. [PubMed]
- Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8; discussion 219. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Nakanishi R, Oka S, Odate S. Video-assisted thoracic surgery major pulmonary resection requiring control of the main pulmonary artery. Interact Cardiovasc Thorac Surg 2009;9:618-22. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Truin W, Siebenga J, Belgers E, et al. The role of video-assisted thoracic surgery in the surgical treatment of superior sulcus tumors. Interact Cardiovasc Thorac Surg 2010;11:512-4. [Crossref] [PubMed]
- Nakanishi R, Fujino Y, Oka S, et al. Video-assisted thoracic surgery involving major pulmonary resection for central tumors. Surg Endosc 2010;24:161-9. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Demmy TL, Yendamuri S, Hennon MW, et al. Thoracoscopic maneuvers for chest wall resection and reconstruction. J Thorac Cardiovasc Surg 2012;144:S52-7. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Muranaka K, et al. Surgical Techniques: Thoracoscopic carinal resection and reconstruction in a patient with mucoepidermoid carcinoma. J Thorac Cardiovasc Surg 2013;145:1134-5. [Crossref] [PubMed]
- Xu X, Chen H, Yin W, et al. Thoracoscopic half carina resection and bronchial sleeve resection for central lung cancer. Surg Innov 2014;21:481-6. [Crossref] [PubMed]
- Lin J, Kang M, Chen S, et al. Surgical Technique: Video-assisted thoracoscopic right upper lobe sleeve lobectomy combined with carinal resection and reconstruction. J Thorac Dis 2015;7:1861-4. [PubMed]
- Li J, Wang W, Jiang L, et al. Video-assisted thoracic surgery resection and reconstruction of carina and trachea for malignant or benign disease in 12 patients: Three centers’ experience in China. Ann Thorac Surg 2016;102:295-303. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Sekhniaidze D, et al. Surgical Technique: Uniportal video-assisted thoracoscopic bronchoplastic and carinal sleeve procedures. J Thorac Dis 2016;8:S210-22. [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: A single centre experience. J Thorac Dis 2016;8:S235-41. [PubMed]
- He J, Wang W, Li J, et al. Surgical Technique: Video-assisted thoracoscopic surgery tracheal resection and carinal reconstruction for tracheal adenoid cystic carcinoma. J Thorac Dis 2016;8:198-203. [PubMed]
- Peng G, Cui F, Ang KL, et al. Surgical Technique: Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Sanders RD. Two ventilating attachments for bronchoscopes. Del Med J 1967;39:170-5.
- El-Baz N, Jensik R, Faber LP, et al. One-lung high-frequency ventilation for tracheoplasty and bronchoplasty: A new technique. Ann Thorac Surg 1982;34:564-71. [Crossref] [PubMed]
- Watanabe Y, Murakami S, Iwa T, et al. The clinical value of high-frequency jet ventilation in major airway reconstructive surgery. Scand J Thorac Cardiovasc Surg 1988;22:227-33. [Crossref] [PubMed]
- Evans E, Biro P, Bedforth N. Jet ventilation. Contin Educ Anaesth Crit Care Pain 2007;7:2-5. [Crossref]
- Rouby JJ, Simonneau G, Benhamou D, et al. Factors influencing pulmonary volumes and CO2 elimination during high-frequency jet ventilation. Anesthesiology 1985;63:473-82. [Crossref] [PubMed]
- Biro P, Layer M, Wiedemann K, et al. Carbon dioxide elimination during high-frequency jet ventilation for rigid bronchoscopy. Br J Anaesth 2000;84:635-7. [Crossref] [PubMed]
- Porhanov VA, Poliakov IS, Selvaschuk AP, et al. Indications and results of sleeve carinal resection. Eur J Cardiothorac Surg 2002;22:685-94. [Crossref] [PubMed]
- Bysani GK, Rucoba RJ, Noah ZL. Treatment of hydrocarbon pneumonitis. High frequency jet ventilation as an alternative to extracorporeal membrane oxygenation. Chest 1994;106:300-3. [Crossref] [PubMed]
- Simes DC. Supplemental jet ventilation in a case of ARDS complicated by bronchopleural fistulae. Crit Care Resusc 2005;7:111-5. [PubMed]
- Woods FM, Neptune WB, Palatchi A. Resection of the carina and main-stem bronchi with the use of extracorporeal circulation. N Engl J Med 1961;264:492-4. [Crossref] [PubMed]
- Torre W, Tamura A, Rabago G, et al. Can the opportunity of cardio-pulmonary bypass be useful in complex general thoracic surgery problems? A report of nine cases. J Cardiovasc Surg (Torino) 2012;53:381-6. [PubMed]
- Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg 1998;65:924-6. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Frasca L, et al. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg 2011;39:1012-7. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: A randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Nakanishi R, Yasuda M. Awake thoracoscopic surgery under epidural anesthesia: Is it really safe? Chin J Cancer Res 2014;26:368-70. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic lobectomy. J Thorac Dis 2013;5:S234-45. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: First report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2 [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-i16. [PubMed]
- Nakanishi R, Shinohara S, Yamashita T, et al. Advances in the use of video-assisted thoracoscopic lobectomy in lung cancer: sleeve bronchoplasty and arterioplasty. Lung Cancer Manag 2014;3:287-95. [Crossref]
- Barclay RS, McSwan N, Welsh TM. Tracheal reconstruction without the use of grafts. Thorax 1957;12:177-80. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Carinal resection for bronchogenic cancer. Semin Thorac Cardiovasc Surg 1996;8:414-25. [PubMed]
- Kaya SO, Sevinc S, Ceylan KC, et al. One-stoma carinoplasty: Right upper sleeve lobectomy with hemicarinectomy for resection of right-tracheobronchial-angle tumors. Tex Heart Inst J 2013;40:435-8. [PubMed]
- Hardin CA, Fitzpatrick MJ. Primary carcinoma of the carina and bifurcation successfully treated by resection and reconstruction. Surgery 1959;46:534-8. [PubMed]
- Mathey J, Binet JP, Galey JJ, et al. Tracheal and tracheobronchial resections: Technique and results in 20 cases. J Thorac Cardiovasc Surg 1966;51:1-13. [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: Results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]
- Poole GV Jr, Meredith JW, Kon ND, et al. Suture technique and wound-bursting strength. Am Surg 1984;50:569-72. [PubMed]
- Takachi T, Shirakusa T, Nakanishi R, et al. Experimental carinal autotransplantation and allotransplantation. J Thorac Cardiovasc Surg 1995;110:762-7. [Crossref] [PubMed]
- Nakanishi R, Hashimoto M, So T, et al. Successful tracheocarinal transplantation. J Cardiovasc Surg (Torino) 1999;40:591-6. [PubMed]
- Shaw KM, Luke DA. Lobectomy with sleeve resection of the bronchus for malignant disease of the lung and the influence of the suture material used for the bronchial repair. Thorac Cardiovasc Surg 1979;27:325-9. [Crossref] [PubMed]
- Grillo HC. Tracheal surgery. Scand J Thorac Cardiovasc Surg 1983;17:67-77. [Crossref] [PubMed]
- McKeown PP, Tsuboi H, Togo T, et al. Growth of tracheal anastomoses: Advantage of absorbable interrupted sutures. Ann Thorac Surg 1991;51:636-41. [Crossref] [PubMed]
- Bush CM, Prosser JD, Morrison MP, et al. New technology applications: Knotless barbed suture for tracheal resection anastomosis. Laryngoscope 2012;122:1062-6. [Crossref] [PubMed]
- Nakagawa T, Chiba N, Ueda Y, et al. Clinical experience of sleeve lobectomy with bronchoplasty using a continuous absorbable barbed suture. Gen Thorac Cardiovasc Surg 2015;63:640-3. [Crossref] [PubMed]
- Matsuoka K, Imanishi N, Yamada T, et al. Clinical results of bronchial stump coverage using free pericardial fat pad. Interact Cardiovasc Thorac Surg 2016;23:553-9. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda L, et al. Prediction of early bronchopleural fistula after pneumonectomy: A multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: An effective method for prevention of postpenumonectomy bronchopleural fistula. Ann Thorac Surg 2005;79:284-8. [Crossref] [PubMed]
- DiMario M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Xu X, Chen H, Yin W, et al. Initial experience of thotacoscopic lobectomy with partial removal of the superior vena cava for lung cancers. Eur J Cardiothorac Surg 2015;47:e8-12. [Crossref] [PubMed]
- Wagner OJ, Hagen M, Kurmann A, et al. Three-dimensional vision enhances task performance independently of the surgical method. Surg Endosc 2012;26:2961-8. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
Cite this article as: Nakanishi R, Oda R, Sakane T, Kawano O, Okuda K, Haneda H, Moriyama S. Video-assisted thoracoscopic surgery (VATS) for central airway tumors: VATS carinal resection and reconstruction. Video-assist Thorac Surg 2018;3:7.


