
Definition of an advanced lung cancer and associated survival after video-assisted thoracoscopic surgery resection
Despite all efforts being undertaken by thoracic oncologists in the last two decades, lung cancer (LC) is still the “oncological killer #1” in the world. Of 1.8 million LC cases diagnosed annually, more than 75% are diagnosed at the advanced stages (1). However, the definition of “an advanced lung cancer” is unclear, especially considering surgical treatment. Some authors define advanced cancer as stages III and IV of the disease (2). Others distinguish early LC (stage I) from an advanced (stages II–IV) one (3). Definition of M. Hennon et al. [2011] for advanced clinical stage of non-small cell lung cancer (NSCLC), used in their article, includes “tumors ≥4 cm, T3 or T4 tumors (based on the American Joint Committee on Cancer, 6th edition), and/or tumors that received neoadjuvant chemotherapy” (4). There are others, who use different criteria: size (more then 3, 4 or 5 cm), T3, T4, preoperative treatment, “central location”, “invasion into adjacent structures” (5). For a surgeon who prefers video-assisted thoracoscopic surgery (VATS) approach, identification of specific problems, which would make the surgery more difficult, is well understood. But the lack of consensus in the definition makes data comparison very complicated and incomparable with the traditional thoracic oncology studies.
According to ESMO Clinical Practice Guidelines 2017 stages I and II are considered “early stages”, stage III is “a locally advanced stage” (LA) that could be divided into a resectable LA-NSCLC and an unresectable LA-NSCLC (6). Stage IV in this scale is supposed to be classified as a systemic (or disseminated) disease.
So, the locally advanced lung cancer corresponds to a tumor that extends to adjacent organs and structures (T3, T4) and/or involves ipsilateral mediastinal lymph nodes (LN). Under these circumstances, the surgically clear margin might be achieved only by either resection of the adjacent tissue together with the pulmonary parenchyma, or might not be achieved at all.
Surgery for unresectable LA-NSCLC
Selective papers describe extra aggressive surgery as part of a combined treatment for IIIB–IV stage (7). Most of them are case reports or short case series with weak or lacking survival data (8,9). There are few papers with long-term results that describe the surgery as a part of trimodality treatment with relatively acceptable outcomes (10,11). Many of those papers are reports on salvage surgery, or urgent treatment of the LC complications as a life-saving procedure (12). Sometimes surgeons intentionally perform curative procedures for stages IIIB and IV such as in rare situations with intrapleural chemotherapy for pleural carcinomatosis, or in cases of unexpected intraoperative upstaging when undertaking surgery for T4N0M0 a patient is suddenly discovered to be N2 or M1 (13,14).
Despite systemic therapy as the standard treatment for metastatic cancer, the surgical resection may enhance the survival rate of patients with solitary distant metastases (15,16). Patients who have resectable primary tumor with oligometastases may benefit from surgical resection as a part of “oligometastatic theory” (17,18). But this is out of the scope of our review because pulmonary resection for oligometastatic stage IV NSCLC is a standard procedure.
Obviously, patients with stages IIIB, IIIC and IV of NSCLC are rare subjects for surgery; therefore we actually consider stage IIIA as a locally advanced LC, where surgical treatment improves outcomes and really makes sense.
Nuances of new staging system for NSCLC
Accepting the TNM staging system as the only basis for subdividing the stages, the thoracic surgical oncologist encounters “a problem of stage III”, where heterogeneity is very considerable due to a “variegated” mixing of the descriptors T and N. According to the 8-th TNM classification (19) stage III is divided into IIIA, IIIB and IIIC (Table 1).
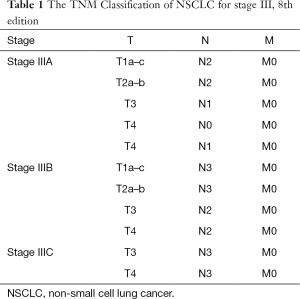
Full table
The most remarkable change in the 8th Edition of UICC TNM Classification for LC is further subdividing of the T descriptor. Even excluding T1 and T2, new subgroups are divided based on the new size intervals: T3—from 5 to 7 cm, and T4—more than 7 cm. The T2 category is also expanded by adding previous T3 classifiers: atelectasis/pneumonitis and/or involvement of the main bronchus, regardless of the distance from the main carina. Invasion of the diaphragm was found to have a similar prognostic impact as other T4 tumors, and has therefore been proposed to be added to this category (20). Thus, if one performs surgery for the tumor more than 7 cm, he needs to realize that this is a potentially IIIB LC.
The N2 disease also raises a number of questions. First of all, N2 disease is also estimated to be heterogeneous, but it has not found any reflection in UICC TNM 8. Technically, mediastinal LNs involvement upstages any disease to at least stage III. L.A. Robinson et al. in 2007 proposed subdivision of N2 disease to “incidental” N2, micrometastatic N2, single station N2 and multiple station/bulky N2-disease (21). It sounds quite reasonable from the surgical point of view. As demonstrated by R. Cerfolio et al. [2008] and T. Tsitsias et al. [2014], the results of surgery for single station N2 or “incidental” N2 are better compared to other N2 types, and adjuvant treatment for specific N2 provides the same long-term results as a neoadjuvant treatment (22,23). At the same time, the 2nd ESMO consensus conference on locally advanced stage III NSCLC asserts that this classification has lost some of its former clinical importance as a treatment guidance (24). The authors use similar, but a more circumstantial treatment algorithm, where “unforeseen” “potentially resectable” and “unresectable” N2 is subdivided (24,25).
Most feels that bulky disease is non-surgical. In other cases of N2, a clear margin could be reached by a standard surgical resection with a systematic LN dissection. This procedure is not an advanced surgery because it does not require resection of adjacent organs. This simple and feasible procedure can be performed by VATS. Results of VATS anatomical pulmonary resections for the patients with N2 have been published during the last 5–10 years (26,27).
Series of VATS lobectomies for “locally advanced lung cancer”
Randomized controlled trials for the use of VATS for LA NSCLC have not been conducted yet. The Cochrane’s database does not contain any systematic review on this topic, either. However, there are several relatively big series with groups of patients in advanced stages treated by VATS (Table 2).
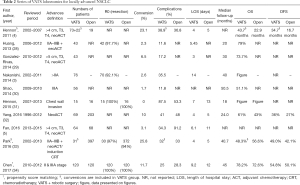
Full table
One of the first published articles for VATS treatment of LA-NSCLC came from a group from Roswell Park Cancer Institute in Buffalo (4). The authors compared VATS and open surgery for clinically advanced LC and found no statistically significant difference in overall survival (OS) (43.7 vs. 22.9 months; P=0.59), disease-free survival (DFS) (34.7 vs. 16.7 months; P=0.84) as well as in percent of postoperative complications. The only difference between groups was that “higher percentage of patients who underwent thoracoscopic lobectomy were able to receive adjuvant therapy compared to the open treatment group (37.2% vs. 5.2%; P=0.006)”. At the same time authors emphasize the limitations of the study: retrospective design and some bias. Moreover, the definition of LA NSCLC was different from the modern one. In addition, the data for the article was collected between 2002 and 2007, at the beginning of the minimally invasive era in thoracic surgery.
R. Nakanishi et al. presented data of 100 consecutive VATS lobectomies (3). They performed broncho- and angioplasty, resection of adjacent organs and tissues, and showed a 3-year survival for stage III in 36.2% of patients. In conclusion, the authors have found VATS resections feasible for the selected patients with locally advanced NSCLC: those with minimal invasion of adjacent organs, no superior sulcus tumor with invasion of the first rib, and no bulky or multiple-station N2 disease. Of note, the data of this study was not classified, meaning the different stages were mixed, and the real LA-NSCLC accounted only for 43.4% of the cases.
Most of the recent works have been feasibility studies. Thus, in an interesting paper by W. Shao et al. [2014] 50 patients with stage IIIA (80% N2) were reported, who underwent VATS lobectomy during 1 year. Authors performed a wide assortment of advanced procedures, and had 5-year survival in 51.1% of patients (30). Despite impressive survival data, patients did not get neoadjuvant chemotherapy, but just postoperative treatment.
Earlier, the same group from Guangzhou, led by professor J. He, demonstrated feasibility of VATS-lobectomies after neoadjuvant treatment for stages IIIA and IIIB (28). The survival data were presented for 1, 2 and 3 years and included 43 patients, 5 of which had stages IIA or IIB.
Another Chinese group from Beijing published matched analyses of the thoracoscopy and thoracotomy for LA-NSCLC (34). They collected data from the prospective database and found 524 patients, and excluded those who underwent bilobectomy, pneumonectomy or sleeve lobectomy and others. In addition, they included stage II patients. After matching 120 cases both in open and VATS group, they found no differences in OS and DFS among the groups. At the same time, despite including different stages in one group, authors presented comparison by clinical stages, and found no differences between VATS and thoracotomy groups in 5-year DFS (stage IIIA 44.2% vs. 30%, P=0.386) and OS (stage IIIA 48.4% vs. 41.4%, P=0.291).
A large study from the Duke University compared VATS and open surgery after induction therapy (32). They demonstrated a trend towards improved OS in VATS group compared to the open one, but the difference was not statistically significant [hazard ratio (HR), 0.56; 95% CI, 0.32–1.01; P=0.053]. The authors openly discussed some limitations of their work. They investigated cohorts of patients with substantial differences between VATS and open groups in terms of stage. But after multivariable adjustment, there was no significant difference in recurrence-free survival between lobectomy groups (HR, 0.68; 95% CI, 0.42–1.09; P=0.11) as well as in recurrence-free 3-year survival (34% vs. 24%) after propensity score matching.
Many feasibility studies were published by surgeons who practice uniportal VATS (u-VATS). There is a comparative study for u-VATS and thoracotomy for LA-NSCLC, including the patients with stage IIB, IIA, and even stage I (4). Less than 30% of patients with stage III were reported in this article.
The most productive u-VATS groups, led by Dr. D. Gonzalez-Rivas, have published a lot of feasibility articles and showed the feasibility of the u-VATS lobectomy in difficult and advanced cases (29). The authors reported a conversion rate of 6.5%, complication rate of 14% and 30-month survival rate of 74% for 43 advanced NSCLC patients who underwent uniportal thoracoscopic resection. Complication rates were similar between early stage and advanced stage patients, suggesting VATS is feasible for more advanced disease.
B. Park et al., presented data with minimally invasive treatment (including robotic assisted lobectomies) of NSCLC after induction chemotherapy (33). They analyzed 31 patients treated with minimally invasive surgery (MIS) approaches (17 robotic and 14 VATS) and 397 treated with thoracotomy for the stage IIB–IIIA LC after neoadjuvant treatment. Reported 3-year OS was 48.3% in the MIS group and 56.6% in the thoracotomy group (P=0.84); the corresponding 3-year DFS were 49.0% and 42.1% (P=0.19). R0 resection rate and postoperative morbidity were also similar in the both groups.
A specific problem of VATS lobectomy is a risk of bleeding and conversion to the open procedure (35). Obviously one should suggest that advanced stage might be accompanied by a higher conversion rate. Patients with clinically node-positive disease have higher chance of conversion during VATS lobectomy (36). However, univariate or multivariate analysis showed that clinical node status did not predict a higher complication rate by in this study. In contrast, the age, decreasing FEV1, prior chemotherapy, and congestive heart failure were significant predictors of morbidity in multivariable analysis. The authors underscored the retrospective design of the study: only 9% of resected tumors were larger than 5 cm. However, since the number of included cases was large enough, 83 of patients had T >5 cm. Similar data on LNs involvement as a predictor of conversions to open have been reported in other publications (37). However, for the countries with a large proportion of the patients with pulmonary tuberculosis, calcified LNs, even tumor negative, may be very dangerous due to their extremely dense adhesions to the segmental PA branches and to the bronchus (38).
Advanced VATS-surgery for lung cancer
The main limitation of most cited articles is an unclear definition of locally advanced LC. As we stated in the beginning of this review, oncologically proven definition of LA-NSCLC is stage III. According to this definition, published works contain from 25% to 40% real LA-NSCLC. The other patients with “centrally located tumors” or “hilum involvement”, or “tumor more than 3 cm”, or “node-positive” cannot be referred to LA-NSCLC group. With that type of non-standard and imprecise descriptions, open letters of some surgeons asking “is video-assisted thoracic lobectomy safe and successful for locally advanced non-small cell lung cancer?” cannot be discussed seriously (39) and allow the opponents to criticize VATS surgery as very elective procedure.
Nowadays, VATS anatomical pulmonary resections for NSCLC have become widely accepted. They are even approved as the surgical treatment of choice for the stage I and II (40-42). At the same time according to the technical features of VATS, some specific procedures become very challenging. Sleeve broncho- and angioplasty, chest wall resection, Pancoast tumor, SVC resection, carina resection, resection and repair of diaphragm comprise an incomplete list of the difficult procedures, which might be called “advanced VATS surgery for lung cancer” (31,43-46).
Advanced or challenging surgery does not necessarily mean it is used for an advanced stage of the disease. Actually, double-sleeve lobectomy is often performed for the stage II NSCLC. As previously described, atelectasis or pneumonitis as well as involvement of main bronchus, irrespective of distance to main carina is defined as T2 by the 8-th TNM. In the case of N0 these patients have to be classified as having non-advanced lung cancer stage I, but who can say that broncho angioplasty is not an advanced procedure?!
The group from Innsbruck has demonstrated advanced VATS procedures and named them “Extended minimally invasive lung resections” (47). They have performed bilobectomy, pneumonectomy and bronchoplasties in 29 out of 370 patients (7.8%), but most of the patients were in stages I and II and just 6 in stage IIIA. This is another illustration that advanced surgery can be performed for non-advanced stages. And vice versa, concerning N2 disease, if one accepts that all pulmonary resections for LC should be accomplished by ipsilateral mediastinal lymphadenectomy, the surgery for selected IIIA (N2) stages will be considered a standard (non-advanced) procedure.
VATS surgery of lung cancer as the way to reduce perioperative immunosuppression and trauma
Theoretically, VATS approach for LC may allow for a better preservation of the patient’s immunity and optimize long-term survival (48). The potential advantages of minimally invasive major pulmonary resection may be explained by various mechanisms. Better preservation of immune function results in less acute inflammatory response, improvement of tumor immune-surveillance, favorable balance of pro-and antiinflammatory cytokines, immunomodulatory cytokines, circulating T (CD4) and natural killer (NK) cells (49-51). For instance, investigators from Korea discovered that VEGF release after surgery may have undesirable effects on residual tumor cells, and could promote tumor growth and metastasis formation (52). This idea inspired a Chinese group to evaluate the levels of VEGFR after VATS and open lobectomies (53). The authors have concluded that VATS approach resulted in relatively stronger antiangiogenic response in the early postoperative period in comparison with the thoracotomy approach. Despite the exclusion of patients with the advanced stage of NSCLC, the authors speculated that less impact of VATS on the level of anti-angiogenic factor sVEGFR2 may be one of the potential mechanisms supporting advantages of VATS lobectomy.
The association between a better preserved immune status following minimal invasive VATS resection for early stage NSCLC and improved survival remains a fantasy for the skeptics, as emphasized by C. Ng and K. Lau (48). In order to demonstrate such a relationship, authors suggested a large randomized trial that measures a comprehensive range of postoperative immune markers and a long-term survival following VATS and open lobectomy.
Chemotherapy is an integral part in the treatment of LA-NSCLC because it improves survival in all subgroups of patients. Multiple studies have shown that chemotherapy is better tolerated after VATS lobectomy than after thoracotomy (3). R. Petersen et al. analyzed 100 consecutive patients treated for lung cancer with lobectomy and adjuvant chemotherapy, and reported that patients undergoing VATS lobectomy had significantly fewer delayed (18% vs. 58%; P<0.001) and reduced (26% vs. 49%; P=0.02) doses. In addition, 61% of the patients undergoing VATS lobectomy received 75% or more of their planned adjuvant therapy versus 40% in the thoracotomy group (P=0.03) (54). Similar results were reported by the China Clinical Trials Consortium where 62 out of 67 patients who underwent VATS resection were able to receive all 3 doses of adjuvant chemotherapy comparing to 53 of 67 of those after thoracotomy (P<0.01) (55).
Interestingly, VATS approach seems to allow for an increase in numbers of LC patients who can be offered surgical treatment. Analyzing high risk (HR) group of patients with NSCLC, L.L. Donahoe et al. [2017], found no significant difference in overall or pulmonary complications when HR patients resected by VATS were compared with the standard risk (SR) VATS group. Moreover, OS was significantly lower for HR patients who had an open operation compared with VATS lobectomy or SR open (P=0.0028) (56). Together with the novel immunological findings and early delivery of the adjuvant treatment, it the oncologic benefits of VATS lobectomy may go beyond mere cancer removal.
Conclusions
The time has come for thoracic surgeons to stop thinking of VATS as a specific surgical technique that fits only for treatment of small peripheral lesions. Many series and trials have demonstrated that VATS anatomical pulmonary resections are a better alternative to open surgery for treatment of NSCLC at any stages, with acceptable size and location.
The patients with advanced stages could benefit from MIS because of a reduced trauma is associated with a reduced immune suppression lowering chances for further spread of metastases. Another argument in favor of VATS is that VATS resection will be tolerated by those LC patients that would not tolerate an open surgery.
Advanced LC is equal to LC of stage III. We should not change the term “advanced LC” because it is accepted by the majority of thoracic oncologists, but it would be worth to use the terms “advanced VATS procedures”, “expert VATS procedures”, or “extended VATS procedures” as technical descriptions. It seems to be necessary to organize multi-institutional trial with strict criteria for including the patients with the stage III lung cancer, who underwent VATS major pulmonary resections, in order to generate sufficient and reliable analytical data for this topic.
Acknowledgments
The author thanks his PhD students Olga Maslak, Marina Tovbina and Alexander Kovalenko for their help in preparation of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.01.04). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66. [Crossref] [PubMed]
- Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis 2014;6:S217-23. [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Fan J, Yao J, Wang Q, et al. Safety and feasibility of uniportal video-assisted thoracoscopic surgery for locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:3543-50. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5. [Crossref] [PubMed]
- Osaki T, Sugio K, Hanagiri T, et al. Survival and prognostic factors of surgically resected T4 non-small cell lung cancer. Ann Thorac Surg 2003;75:1745-51; discussion 1751.
- Yang HX, Hou X, Lin P, et al. Survival and risk factors of surgically treated mediastinal invasion T4 non-small cell lung cancer. Ann Thorac Surg 2009;88:372-8. [Crossref] [PubMed]
- Stamatis G, Eberhardt W, Stüben G, et al. Preoperative chemoradiotherapy and surgery for selected non-small cell lung cancer IIIB subgroups: long-term results. Ann Thorac Surg 1999;68:1144-9. [Crossref] [PubMed]
- Stupp R, Mayer M, Kann R, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009;10:785-93. [Crossref] [PubMed]
- Farbicka P, Nowicki A. Palliative care in patients with lung cancer. Contemp Oncol (Pozn) 2013;17:238-45. [Crossref] [PubMed]
- Baba T, Uramoto H, Kuwata T, et al. Intrapleural chemotherapy improves the survival of non-small cell lung cancer patients with positive pleural lavage cytology. Surg Today 2013;43:648-53. [Crossref] [PubMed]
- Hu R, Jiang H, Li H, et al. Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS. J Thorac Dis 2017;9:1317-21. [Crossref] [PubMed]
- Yamanaka R. Medical management of brain metastases from lung cancer Oncol Rep 2009;22:1269-76. (Review). [Crossref] [PubMed]
- Schuchert MJ, Luketich JD. Solitary sites of metastatic disease in non-small cell lung cancer. Curr Treat Options Oncol 2003;4:65-79. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Niibe Y, Chang JY, Onishi H, et al. Oligometastases/Oligo-recurrence of lung cancer. Pulm Med 2013;2013:438236 [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 8th edition. Oxford: John Wiley & Sons, 2016.
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:243S-65S.
- Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7. [Crossref] [PubMed]
- Tsitsias T, Boulemden A, Ang K, et al. The N2 paradox: similar outcomes of pre- and postoperatively identified single-zone N2a positive non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:882-7. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89-98. [Crossref] [PubMed]
- Li Y, Wang J. Comparison of clinical outcomes for patients with clinical N0 and pathologic N2 non-small cell lung cancer after thoracoscopic lobectomy and open lobectomy: a retrospective analysis of 76 patients. J Surg Oncol 2012;106:431-5. [Crossref] [PubMed]
- Wang S, Zhou W, Zhang H, et al. Feasibility and long-term efficacy of video-assisted thoracic surgery for unexpected pathologic N2 disease in non-small cell lung cancer. Ann Thorac Med 2013;8:170-5. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Shao W, Liu J, Liang W, et al. Safety and feasibility of video-assisted thoracoscopic surgery for stage IIIA lung cancer. Chin J Cancer Res 2014;26:418-22. [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5.
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-76.e2. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20; discussion 520-1. [Crossref] [PubMed]
- Li Y, Wang J. Analysis of lymph node impact on conversion of complete thoracoscopic lobectomy to open thoracotomy. Thorac Cancer 2015;6:704-8. [Crossref] [PubMed]
- Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014;6:S623-30. [PubMed]
- Cioffi U, De Simone M, Baisi A. Is video-assisted thoracic lobectomy safe and successful for locally advanced non-small cell lung cancer? J Thorac Cardiovasc Surg 2013;146:1302-3. [Crossref] [PubMed]
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Dziedzic D, Orlowski T. The Role of VATS in Lung Cancer Surgery: Current Status and Prospects for Development. Minim Invasive Surg 2015;2015:938430
- Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer 2016;100:114-9. [Crossref] [PubMed]
- Zhou S, Pei G, Han Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Ruffini E, et al. Corrigendum to “A comparative analysis of Pancoast tumour resection performed via video-assisted thoracic surgery versus standard open approaches” Interact Cardiovasc Thorac Surg 2016;22:121. [Interact CardioVasc Thorac Surg 2014;19(3):426-435]. [Crossref] [PubMed]
- Augustin F, Maier H, Lucciarini P, et al. Extended minimally invasive lung resections: VATS bilobectomy, bronchoplasty, and pneumonectomy. Langenbecks Arch Surg 2016;401:341-8. [Crossref] [PubMed]
- Ng CS, Lau KK. Surgical trauma and immune functional changes following major lung resection. Indian J Surg 2015;77:49-54. [Crossref] [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [Crossref] [PubMed]
- Ng CS, Lee TW, Wan S, et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J Invest Surg 2005;18:81-8. [Crossref] [PubMed]
- Ng CS, Whelan RL, Lacy AM, et al. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg 2005;29:975-81. [Crossref] [PubMed]
- Lee SH, Jeong D, Han YS, et al. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 2015;89:1-8. [Crossref] [PubMed]
- Peng J, An S, Wang HP, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer versus thoracotomy: a less decrease in sVEGFR2 level after surgery. J Thorac Dis 2016;8:323-8. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Zhi X, Gao W, Han B, et al. VATS lobectomy facilitates the delivery of adjuvant docetaxel-carboplatin chemotherapy in patients with non-small cell lung cancer. J Thorac Dis 2013;5:578-84. [PubMed]
- Donahoe LL, de Valence M, Atenafu EG, et al. High Risk for Thoracotomy but not Thoracoscopic Lobectomy. Ann Thorac Surg 2017;103:1730-5. [Crossref] [PubMed]
Cite this article as: Pischik VG. Definition of an advanced lung cancer and associated survival after video-assisted thoracoscopic surgery resection. Video-assist Thorac Surg 2018;3:6.


