
Immune effects after uniportal nonintubated video-thoracoscopic operations
Introduction
It is well and consolidated belief that surgical injury may lead to transient depression of the immune function that can potentially cause infective complications as well as cancer spread (1,2). The biological basis of this statement might likely be due to both surgical and anesthesiological proceedings (3,4). Among all immunological effectors the lymphocytes represent one of the most important elements and namely, natural killer cells (CD16/CD56) play a key role against tumors and infections thank to their cytotoxic activity (5,6). General anesthesia and especially one lung ventilation combined with surgical stress and extensive tissue trauma affect negatively the response lymphocytes and natural killer population (7-9). These effects are accomplished by decreasing the basal cytotoxicity action, predisposing to post-operative alteration of wound healing processes (10), facilitating infections and increasing the risk of tumor progression and metastases (11). On the other hand, a minimization of surgical trauma in thoracic surgery demonstrated to preserve the immune defense (12).
The increasing evolution of non-intubated thoracic surgery allowed the execution of progressively more elaborate operations in patients with different pathologies (13). Nonintubated surgery revealed a potential in reduction of inflammation (14), a better preservation of immunological function (15) compared to the traditional approaches. Our program of non-intubated thoracic surgery named the Awake Thoracic Surgery Research Group was specifically created for this purpose by one of us (Tommaso Claudio Mineo), who is still the main mentor and coordinator (16). To date, more than one thousand non-intubated procedures have been carried out in our department (13). Early operations were done under epidural anesthesia and three-port video-assisted thoracic surgery (VATS) (17) but starting from 2005, operations have been preferably accomplished through a unique thoracoscopic access under intercostal block and non-intubated anesthesia (18).
In this study our aim is to analyze the variations in the different immune patterns after uniportal VATS operations under nonintubated anesthesia compared to an intubated group that in the same period underwent similar procedures.
Methods
From December 2005 to December 2016, a total amount of 878 patients underwent uniportal VATS operations under nonintubated anesthesia. This combined approach was applied to a variety of nononcologic (pneumothorax, diffuse and bullous emphysema, pleural infection and interstitial lung disease) and oncologic conditions (pleural effusion, peripheral lung nodules and mediastinal masses) (13,18) including both minor (biopsies and wedge resections) as well as major (decortications, segmentectomies and lobectomies) operations.
Inclusion criteria for nonintubated surgery were generic indications to VATS surgery such as normal mass body index <30 kg/m2, no imaging or clinical suspect for obliterated pleural cavity and hemodynamic stability, combined with patient’s cooperation and absence of excessive anxious attitude.
Indeed, all patients released written fully informed consent after having listened, read and understood the explanations of the main details including theoretical pros and cos of nonintubated operations. In particular, the form advised that during a nonintubated procedure, surgical maneuvers might be somewhat demanding and less tolerated with the risks of hypercapnia and intolerance. Conversely, the immediate postoperative course was predicted to be smoother than that after intubated procedures given the absence of weaning-related side effects.
Technique
During the procedure patients were continuously monitored by pulse oximeter, arterial blood gases, body temperature, electrocardiogram, systemic and central venous blood pressure, bispectral index and end-tidal CO2. A 5-mL solution of 2% lidocaine was aerosolized for 5 min, before the procedure, in order to prevent cough reflex. Furthermore, the patient inhaled O2 during the operation through a ventimask to maintain saturation greater than 90%.
Uniportal procedures were done by intercostal block with separate infiltration of lidocaine 2% (4 mg/kg) and ropivacaine 7.5% (2 mg/kg). Intraoperative intravenous administration of benzodiazepine (midazolam 0.03–0.1 mg/kg) or opioids (remifentanil 15 µg/kg/min) allowed the patients to tolerate all of the intrathoracic phases. Unexpected anxiety or panic occurring intraoperatively were sedated without interfering with spontaneous breathing by increasing propofol (0.5 mg/kg) continuous infusion.
At the end of the procedure one 28# chest tube was collocated at the posterior limit of the surgical wound. Drinking, eating, and walking was generally allowed in the same day of surgery. Patients were discharged after radiological evidence of complete lung re-expansion, limited pleural effusion (no more than 100 mL/day), and no air leakage. Patients with protracted air leakage (>5 days) were discharged with a Heimlich valve.
Laboratory assessment
Venous blood samples were taken in the morning prior the operative session from antecubital vein, and it was repeated at postoperative days 1, 7, and 14. The Laboratory of Onco-hematology of our institution performed the immediate real-time tests without need of storage. Total lymphocytes count was assessed with a cell counter (Coulter Beckmann, MedLab, Cupertino, CA, USA). Lymphocyte-subset were analyzed with FACSCanto II esa-color flow cytometry (BD Biosciences, San Diego, CA, USA) using monoclonal antibodies specific to the cell markers. Before, samples were incubated with monoclonal antibodies and then processed with the erythrocyte lyse-wash technique (ammonium-chloride solution 1×; BD Biosciences). The identification of phenotypes of lymphocyte population was made by anti-CD3-FITC, anti-CD4-APC-H7, anti-CD8-PE-Cy7, anti-CD56(3-)-PE, anti-CD19-APC, and anti-CD45-PercPCy5.5 (BD Biosciences).
Study design and statistics
The study was designed as retrospective and approved by our Institutional Review Board, which allowed the review of all laboratories values. We were able to retrieve data about lymphocyte subpopulations for 542 patients. Clinical features of this cohort of patients are summarized in Table 1. Furthermore, only on observational basis we compared these data to those measured in 106 patients, who had been scheduled for nonintubated procedure in the same period but who refused this option preferring intubation and general anesthesia. Statistical analysis was performed with the SPSS software package (SPSS® 18 version, Chicago, IL, USA). Data were expressed as mean and standard deviation. Significant level was considered P<0.05. Non-parametric tests were prudentially preferred using Wilcoxon for within group and Kruskal-Wallis for between-group evaluations, respectively. Survival analysis was conducted with the Kaplan-Meier method and significance between group was assessed with the log rank test.
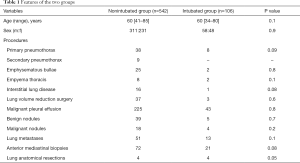
Full table
Results
As shown in Table 1, all main variables resulted homogeneously distributed between groups. Postoperative results of the patients are shown in Table 2. We did not experience intraoperative mortality. Global time spent in the operative room including induction and awake time was significantly longer in intubated group (85±41 vs. 118±34 minutes; P=0.03), whereas operative time was still shorter in the intubated group (68±29 vs. 53±41 minutes) yet not significant.

Full table
Immunological impact
Postoperative immunologic trends are shown in Figure 1 and Table 3. As expected, total leukocytes count increased after surgery in both groups. The total lymphocytes count showed a lesser drop in the nonintubated group in both post-operative day 7 (P=0.04) and post-operative day 14 (P=0.05), with the nonintubated group also displaying a nearly-significant more rapid restoration of the baseline value. Among the subpopulations in the nonintubated group, there was a significant lesser reduction of natural killer lymphocytes at 7 (P=0.03) and 14 (P=0.04) days following the procedure compared to the intubated group (Figure 1). On the other hand, the other subpopulations did not present significant difference between groups.

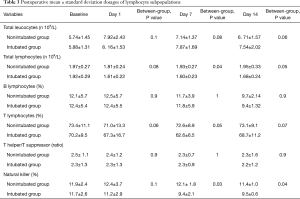
Full table
Morbidity
We had 5 deaths (0.9%) within 30 days from the operation, which was significantly lesser than the 5 deaths (4.7%; P=0.04) occurred in the intubated group. Major morbidity rate was significantly higher in the intubated group 52 (9.5%) vs. 20 (19%) (P=0.03). The favorable ratio was mainly due to the lower number of postoperative pneumonia: 27 patients (4.9%) in the nonintubated group versus 13 patients (12%) in the intubated one (P=0.01). This more uncomplicated postoperative course had an impact on mean hospital stay (4.2±2.8 vs. 6.1±2.6 days), which was significantly faster in the nonintubated group (P=0.04).
Long term effects in malignant effusion
We also evaluated long term survival in a consistent cohort of patients with malignant pleural effusion, which represents the vast majority of the neoplastic pathologies operated. The subset included 217 patients undergoing a nonintubated procedure and 43 patients an intubated procedure, respectively. Survival rate was significantly higher in the nonintubated group (P=0.03) (Figure 2).
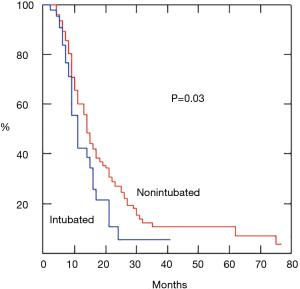
Discussion
Lymphocytes, and mainly natural-killer cells (CD16+/CD56+), are important effectors of the immune response (5). This action largely depends on their cytotoxic activity as well as on their capability to release regulatory cytokines (6). Traditional intubated surgery (10) especially under one-lung ventilation (8,9) may create many significant side effects in the immunologic sphere decreasing basal natural-killer cytotoxicity. In addition, surgical stress and the extensive tissue trauma may reduce the amount of circulating lymphocytes and namely the rate of natural-killer cells (3). As a result, the impairment of the immune function may favor post-operative infections and hinder postoperative healing processes (19). Furthermore, reduced cytotoxic activity of peripheral-blood lymphocytes may increase the risk of tumor progression and metastatic spread (11). Even though lessened by the uniportal approach (20) and one-lung ventilation might evoke a cascade of many oxidative changes, eventually resulting in a compartmental release of pro-inflammatory mediators (19,21,22) including interleukin 6 (23).
As far as we know, our program of awake VATS operations is the oldest surgical program specifically created for this purpose. In many studies we demonstrated that nonintubated thoracic surgery allows a faster recovery, with shorter hospital stay and lesser economical expenses (13-18). In the present one, we found that the nonintubated procedure can reach successful results with a significantly lower 30-day mortality and major morbidity rates. We would also remark the lower rate of postoperative pneumonia that is directly connected to bilateral lung ventilation and the lack of re-expansion after one lung ventilation (16). As a result, we reported a lesser duration of the hospital stay. At the same time, we experienced a significantly lower decrement of natural killer lymphocytes at day 7 and 14 in the nonintubated group and bilateral lung ventilation may explain this trend.
Spontaneous bilateral ventilation might have also improved the oncological outcomes. The frequent postoperative onset of undiagnosed metastases may be likely due to the rapid growth of occult metastases to the lack of immune control related to postoperative immunologic depression (6,24,25). In a previous study, we did not report significant differences in postoperative survival among patients undergoing colorectal pulmonary metastasectomy (26). This finding can be probably explained with the short sample size of the study groups. On the contrary, the positive effect of nonintubated surgery could now be visible on the larger population of patients with malignant pleural effusion.
The present study presents obvious and crucial limitations. First of all, the non-randomized nature of the two study groups and the absence of a proper propensity score approach. Second, the group allocation based on patient’s preference. Third, the peculiar features of the neoplastic patients extrapolated among those with advanced disease and influenced by a so heterogeneous number of interfering factors. Fourth, the lack of similar comparison for patients with other neoplastic disease, unsuitable because of scant sample size. Fifth, the study is restricted to lymphocyte subpopulation, whereas immunological spectrum is so wide and would require the analysis of more factors.
Despite these evident flaws, we think that this observational study can provide interesting information in a consistent sample size. According to these supplemental data about immunological effect of nonintubated surgery we would endorse the diffusion of this kind of anesthesia especially in neoplastic conditions.
Conclusions
In the last decades, increasing attention has been dedicated to the importance of immune-competence in the postoperative period. Uniportal operations under nonintubated anesthesia demonstrated a significant lower impact on immunological response compared to the uniportal procedures in general anesthesia with selective intubation and one-lung-ventilation. This may have effects on both postoperative 30-day mortality and morbidity with a significant shorter hospital stay. We also found a significant influence on long-term survival in a consistent subset of patients operated for malignant pleural effusion.
Acknowledgments
We thank so much Daniela Fraboni from the Laboratory of Onco-hematology of our institution for her precious and qualified cooperation in evaluating the blood samples.
Funding: This research was supported by the Italian Health Ministry (title of the project: ‘Pro lo genetico associato al fenotipo metastatico e alla prognosi nei tumori polmonari’).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Non-intubated Thoracic Surgery”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.01.02). The series “Non-intubated Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. TCM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was submitted and approved by the Internal Review Board at Tor Vergata University of Rome with the authorization code 628/15. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meakins JL. Surgeons, surgery, and immunomodulation. Arch Surg 1991;126:494-8. [Crossref] [PubMed]
- Griffis CA, Page G, Kremer M, et al. Implications of immune function to anesthesia care. AANA J 2008;76:449-54. [PubMed]
- Marik PE, Flemmer M. The immune response to surgery and trauma: implications for treatment. J Trauma Acute Care Surg 2012;73:801-8. [Crossref] [PubMed]
- Whitehead T, Slutsky AS. The pulmonary physician in critical care: ventilator induced lung injury. Thorax 2002;57:635-42. [Crossref] [PubMed]
- Herberman RB, Ortaldo JR. Natural Killer cells: their role in defenses against disease. Science 1981;214:24-30. [Crossref] [PubMed]
- Imai K, Matsuyama S, Miyake S, et al. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000;356:1795-9. [Crossref] [PubMed]
- Tønnesen E, Hohndorf K, Lerbjerg G, et al. Immunological and hormonal responses to lung surgery during one-lung ventilation. Eur J Anaesthesiol 1993;10:189-95. [PubMed]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. [Crossref] [PubMed]
- Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. [Crossref] [PubMed]
- Kutza J, Gratz I, Afshar M, et al. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg 1997;85:918-23. [Crossref] [PubMed]
- Lee HH, Kang H, Cho H. Natural killer cells and tumor metastasis. Arch Pharm Res 2017;40:1037-49. [Crossref] [PubMed]
- Koltun WA, Bloomer MM, Tilberg AF, et al. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg 1996;171:68-72. [Crossref] [PubMed]
- Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. [Crossref] [PubMed]
- Mineo TC, Tacconi F. From “awake” to “monitored anesthesia care” thoracic surgery: A 15-year evolution Thoracic Cancer 2014;5:1-13. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery J Thorac Cardiovasc Surg 2012;143:249-50. [Crossref] [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Sellitri F. Non-intubated video-assisted thoracic surgery from multi to uniport approaches: single-centre experience. Eur Med J Respir 2016;4:104-12.
- Baker EA, El-Gaddal S, Williams L, et al. Profiles of inflammatory cytokines following colorectal surgery: relationship with wound healing and outcome. Wound Repair Regen 2006;14:566-72. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Wrigge H, Zinserling J, Stüber F, et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 2000;93:1413-7. [Crossref] [PubMed]
- Funakoshi T, Ishibe Y, Okazaki N, et al. Effect of re-expansion after short-period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokines gene expression in isolated rabbit lung. Br J Anaesth 2004;92:558-63. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Vanni G, et al. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci 2017;18:E1466 [Crossref] [PubMed]
- Ghanim B, Schweiger T, Jedamzik J, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interact Cardiovasc Thorac Surg 2015;21:616-23. [Crossref] [PubMed]
- Abdelnour-Berchtold E, Perentes JY, Ris HB, et al. Survival and local recurrence after video-assisted thoracoscopic lung metastasectomy. World J Surg 2016;40:373-9. [Crossref] [PubMed]
- Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254-61. [Crossref] [PubMed]
Cite this article as: Mineo TC, Ambrogi V. Immune effects after uniportal nonintubated video-thoracoscopic operations. Video-assist Thorac Surg 2018;3:4.


