
Awake video-assisted thoracic surgery resection of lung nodules
Introduction
In 1913, Jacobaeus introduced a technique for dividing pleural adhesions in the awake patient which gained some popularity in the 1940s albeit its usefulness had become limited by the introduction of the antibiotic treatment for tuberculosis (1). Reportedly, pleurolysis was used in the awake patient as part of the management of tuberculosis since the 1920s (2).
Awake thoracoscopy was introduced for surgical diagnosis and treatment of pleural effusions as a routine option in the 1980s (3). Almost two decades later, the indications for awake thoracic surgery also included hyperhidrosis (sympathectomy) (4), pneumothorax (5), initial stages of empyema (6), and pericardial effusions (7). Before 2000, reports of thoracoscopic lung resections for spontaneous pneumothorax had been published (8,9). From the beginning of 2000s there has been an enormous development of new minimally invasive surgical techniques for both minor and major pulmonary resections. The advancements of surgical techniques and instrumentation allowed thoracic surgeons to improve their surgical skills, leading them to explore a field believed insurmountable, such as the one related to the pulmonary resections in non-intubated patient. In 2007, Al-Abdullatief et al. (10) showed the possibility of performing major thoracic surgery with the patient awake or minimally sedated. Later, awake lung surgery was introduced in clinical practice both for the surgical management of lung emphysema (11) and lung nodules (wedge resections and anatomical lung resections) (12,13), whereas there still remain only few cases of scientific reports describing sleeve resections and carinal reconstructions (14), tracheal resections (15), pneumonectomies (16) performed in the awake/nonintubated patient. In Table 1 we have summarized the relevant published papers on nonintubated video-assisted thoracic surgery (VATS) wedge resections of lung nodules.
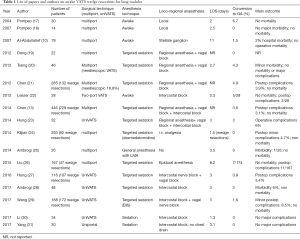
Full table
Awake thoracic surgery offers the unique possibility of reducing postoperative morbidity thus facilitating early discharge from the hospital. The primary goal of the surgeon/anesthesiologist team is to make the operation safe and effective while reducing the psychological distress of the patient. Few anesthesiologists have experience in awake thoracic surgery, most of them with good experience in loco-regional anesthesia (epidural, intercostals blocks) (32). Nevertheless, the administration of anesthetics is not without side effects and risks.
In this paper, we discuss on the use of VATS for awake lung nodule resections, the indications and patient selection, the intra-operative complications, the management of adverse conditions and the choice of conversion to general anesthesia. We also show our experience in developing of non-intubated anesthetic techniques applied to uniportal VATS (UniVATS). Nowadays, the combination of UniVATS and awake surgery is perceived by thoracic surgeons as the final step of Minimally Invasive Thoracic Surgery, a stage leading to fast-tracking in thoracic surgery (33).
What does “awake” really mean?
Awake thoracic surgery includes non-intubated thoracoscopic procedures performed under regional anesthetic techniques in spontaneous breathing and fully conscious patients (34). The regional anaesthetic techniques consist of local anaesthesia, intercostal nerve blocks, intra-pleural block, paravertebral blocks or thoracic epidural anaesthesia (TEA) without the use of any form of sedation or, at the most, with just the so called anxiolysis, as a technique meant to provide minimal sedation (34). “Non-intubated” thoracoscopic procedure is a generic term to indicate a combination of loco-regional anesthetic techniques with a variable level of sedation. In the literature, we could find every form of sedation, ranging from no sedation at all to a non-intubated general anesthesia VATS (35). Therefore, non-intubated thoracoscopic procedure is the correct term to indicate a thoracic procedure in which every form of intubation (endo-tracheal tube, double lumen tube) is precluded. Reviewing the scientific literature, anaesthesia in the—inappropriately called—awake thoracic surgery is performed with different modalities of sedation and various levels of analgesia (36):
- Awake (no sedation, only local anesthetic or analgesia): a wide-awake VATS means only an isolate intercostal block with local anesthesia;
- Minimal sedation (anxiolysis): relief of trepidation or agitation with minimal alteration of sensorium;
- Moderate sedation (conscious sedation);
- Deep sedation;
- The continuum of sedation;
- MAC (monitored anesthesia care) (36).
In our experience, the majority of minor thoracic procedures can be managed by uniportal surgery: in these cases, a single intercostal space block combined with a moderate sedation is usually sufficient to obtain an ideal anxiolysis and analgesia during the surgical procedure.
It is agreed that MAC should be mandatory in major pulmonary resection in non-intubated patients (37-39). Further studies should be done in order to definitively prove whether a minimal sedation in combination with epidural anaesthesia and associated phrenic and vagus nerves blockage are sufficient to complete a major lung resection by VATS.
Indications and patient selection in non-intubated/awake VATS
Modern indications for non-intubated thoracic surgery include conditions in the following structures: (I) pleura/chest wall conditions (pleural effusions, pneumothorax, endothoracic cysts, pericardial effusion, empyema); (II) mediastinum (biopsies, cysts, thymoma, sympathetic chain); (III) lung (emphysema, interstitial lung diseases and lung nodules). Different levels of anesthetic approaches can be adopted in non-intubated patients: from a deep sedation (which requires a MAC) to a loco-regional anesthesia and a simple local anesthesia. Regardless of the anesthetic approach, the success of each different techniques depends on an appropriate patient selection.
Not all patients are fit for awake/non-intubated VATS lung resection. A team composed of chest physicians, thoracic surgeons, and anesthesiologists together must select the right candidate through a careful assessment of risk factors resulting from adverse tracheobronchial conditions (i.e., difficult intubation, OSAS), patient’s comorbidities, personality disorders (i.e., anxiety, level of collaboration, mental impairment) and pre-existing medical issues (i.e., body weight, chest deformities, difficulties in management of locoregional anesthesia, paralysis) (Table 2). Furthermore, the criteria for non-intubated VATS in minor or pleural procedures are different from those in major pulmonary resections and recommendations have been established in patients with increased risk for general anesthesia. In this respect, high-risk patients with pre-existing pulmonary disease and the elderly patients should be the categories of patients which should benefit even more of awake surgery. The success of an awake/nonintubated VATS procedure could be the result of the convergence of the different needs and expectations of surgeon, anesthetist and patient (Table 3).

Full table

Full table
Intra and peri-operative issues in non-intubated/awake VATS
Different issues can arise from a nonintubated/awake VATS resection of lung nodules, depending on the patient (psychological status, comorbidities, body conformation, pain), the invasiveness of the procedure planned (multi/uniport, risk of conversion to thoracotomy), the intra-operative problems (presence of adhesions, localization of nodules, its dimensions, suitable space to work in pleural cavity, surgical margin resections not clear, necessity to explore the mediastinum). Preoperative psychological preparation is mandatory. Patients must be informed about realistic description of the operating room, expected discomforts (patient positioning, transitory pain) and level of co-operation expected, potential risks (conversion to general anesthesia), safety measures (i.e., tilting of the surgical table) and stages of the procedure (downtime waiting for frozen section). Premedication with sedatives and anticholinergics in patients is quite controversial (40), and decisions should be made based on the patient’s clinical conditions and the anesthetic technique. According to the invasiveness of the procedure, anesthetic technique selection in thoracic awake lung VATS resections ranges from local anesthesia (intercostal blocks) to thoracic regional blocks (paravertebral block, serratus anterior plane block) and thoracic epidural anesthesia (TEA) with different levels of sedation to general anesthesia with laryngeal mask airway placement combined with a specific anesthetic protocol that includes a careful monitoring and support of the vital functions (MAC). A regional anesthetic technique is mandatory in thoracic awake lung VATS resection. TEA, paravertebral block and intercostal block all improve postoperatively the respiratory function. Wang et al. (41) reported that intraoperative regional anesthesia, such as multilevel thoracoscopic intercostal nerve blocks or paravertebral blocks, provide a regional anesthetic component and exert an anesthetic-sparing effect. During nonintubated VATS, the surgical pneumothorax obtained from the lung collapse is similar to that of intubated single-lung ventilation. In awake VATS lung resections, an adequate pleural space is required to guarantee any surgical maneuvering. The lung volume will usually decrease to functional capacity, allowed by an adequate adhesiolysis and a gentle manipulation of the lung. The pneumothorax-induced results in a reduction of oxygenation, easily corrected with supplemental oxygen, and a permissive hypercapnia, generated by the development of pressure gradients after the creation of the pneumothorax, usually well tolerated and completely settled immediately after surgery. Some contributions from the literature (42) focused on the use of CO2 insufflation combined with a single lumen endotracheal tube intubation as a feasible and safe airway management in VATS, proving shorter operative times and no complications CO2 related if compared with a traditional double lumen tube intubation. Sporadic experiences in using CO2 insufflation seem to be promising in awake VATS too, in particular for adhesiolysis and in case of excessive movements of mediastinum or diaphragm displacement (unpublished data). The indications, the type of anesthesia monitoring, and the level of CO2 insufflation should be clearly stated.
The main drawbacks of nonintubated VATS, which can preclude even a wedge resection of a peripheral nodule, include coughing and movements of the diaphragm and the mediastinum. Phrenic and vagus nerves are the main causes of the cough reflex. In the minor resections, there is a limited manipulation of the lung and therefore less risk of haemodynamic effects or coughing. Nevertheless, if mediastinum exploration is requested to remove lymph nodes, the risk of coughing is high and the intrathoracic vagal and phrenic nerve blocks are mandatory, with minimal secondary effects (43,44). Further administration of aerosolized lidocaine should guarantee an optimal cough suppression (45) allowing surgeons to smoothly perform a mediastinal dissection and an appropriate lung traction. Chen et al., in two different studies (21), demonstrated that intravenous combined anesthesia under spontaneous breathing did not negatively affect the completeness of lymph node dissection as compared to intubated anesthesia.
Surgeon should also deal with the issues of localizing the pulmonary nodule and ensuring clear resection margins. In the absence of a preoperative diagnosis and if the lesion cannot be readily found, several techniques have been developed to facilitate intra-operative localization of solitary pulmonary nodule (SPN) during VATS (46). Methylene blue injection has been abandoned in favour of specialized equipment, such as CT-fluoroscopy (47) or use of gamma probe, required in case of injection of specific radiotracer. Intra-operative ultrasound detection requires a specific flexible ultrasound probe and it is operator dependent (48). In non-collapsed lung or emphysematous patients, the localization of SPN is also limited. For nodules not amenable to finger palpation, VATS resection after CT-guided hook wire localization for SPN remains the most diffuse method to localize a pulmonary nodule (49). For lesions not in direct contact with the visceral pleura or presenting as ground-glass nodules on low-dose CT imaging, a preoperative CT-guided dye localization is a further option to facilitate tumor identification during VATS (50). More recently a new method to resect localized peripheral lung lesions using fluoroscopy-assisted thoracoscopic surgery (FATS) preceded by CT-guided positioning of fragmented platinum microcoils into or around the lesion (51) has been introduced.
Intraoperative frozen pathology for indeterminate lung lesions is essential to establish the proper extent of resection and whether a lymphadenectomy is required or not. The presence of margins involved by tumour with the attendant choice to opt for an anatomical lung resection could represent a valid reason to convert to VATS in general anesthesia.
The case of the nonintubated/awake UniVATS
In 2004, the Italian contribution on this field (17,52) was seen as a welcome news which could further stimulate the scientific research. UniVATS has been shown to reduce postoperative pain, residual paresthesia and hospital stay compared with conventional multiport VATS (53). These concepts can furthermore be stressed by the absence of general anesthesia. Due to the technologic upgrading in surgical instrumentation, the use of uniportal technique has been increasing steadily and it is the surgical approach of choice for awake VATS. When performing an awake UniVATS approach, we can easily apply a single intercostal space blockade under thoracoscopic view. The advantage of avoiding any form of sedation and applying a regional anesthetic technique (TEA, intercostal nerve blocks) for pain, is evident at the end of procedure in terms of anesthetic drug sparing effect and reduced need for opioids (opioid-free anesthesia); this favourably affects the length of hospital stay and the rapid return to health. High risk patients for general intubated anesthesia, such as elderly patients or those with poor pulmonary function, could beneficiate even more of this minimal invasive approach (34). Nowadays, it appears clear that availability of local expertise and resources along with sound clinical judgement will equally contribute to the decision-making process for non-intubated UniVATS.
In nonintubated UniVATS lung nodule resections, a single intercostal space block is usually sufficient to control the afferent nerves and, consequently, the pain. Because of the limited incision, less-invasive regional techniques are required. Our experience suggests that an additional infiltration of the same intercostal space, laterally to the sympathetic chain, under thoracoscopic view, together with a topic anaesthesia on the mediastinal pleura (near the hilum) and a local infiltration of phrenic nerve (cough sedation), should guarantee a good management of the intraoperative pain, an adequate mediastinum manipulation, avoiding the TEA which requires positioning time, anesthesiologist’ experience and not without any potential complications.
Finally, further perspectives are centred first on the introduction of the subxiphoid approach and the tubeless VATS. The ability to access both thoracic cavities using a subxiphoid incision may reduce the risk of intercostal nerve injury; this feature is even more evident when a bilateral surgical approach is necessary (54). “Tubeless” VATS is one of the newest developments, combining UniVATS plus Awake VATS: patients undergo awake UniVATS without placement of a chest tube. The rationale for this approach results from the observation that every surgical tube or catheter or vascular line forces patients in the hospital; accordingly, every “tube” is removed before patients are transferred to the recovery room. As a consequence, tubeless VATS may contribute to further minimizing the pain and shortening the duration of their hospitalization thereby facilitating “fast-track” thoracic surgery (31).
Management of complications and conversion to general anesthesia
An appropriate intra-operative monitoring is an essential requisite to manage complications. A missed pain control and an inappropriate sedation could determine major risks in surgical manoeuvres and waste of time that ultimately could lead to a general anesthesia conversion. To avoid this event, a combination of a proper regional anesthetic technique (mostly the TEA) with a sedation strategy including a monitoring of the level of consciousness using electroencephalographic analysis by bilateral bispectral index (BIS) (44) should be taken into consideration. This is especially the case of the VATS awake resection of peripheral nodules in which different events could upset the initial surgical plan (difficult research of nodule, waste of time during frozen section, surgical margins involved by tumour requiring an extension of resection, conversion to an anatomical resection, necessity to do a mediastinal lymphadenectomy).
MAC is a specific anesthetic protocol that includes careful monitoring and support of vital functions; during this anesthetic technique, airway management is minimal and non-invasive so that the anesthetist is able to minimize intraoperative complications, acting in a short time on hemodynamic instability, hypercapnia, coughing, pain, nausea, hypothermia.
In the event of surgical complications (limited pleural space caused by excessive movements of the diaphragm and the mediastinum, tenacious pleural adhesions, uncontrolled bleeding or air leaks, operative time extension) it is crucial to have an effective team communication and a plan of conversion ideally to be discussed preoperatively. Both anesthesiologic conversion to general anesthesia and surgical conversion to an extra-port VATS or directly to a thoracotomy, need an experienced anesthesiologist and a skilled surgical team in order to avoid catastrophic events.
Overall, no published data show a significant difference in operative morbidity if they compare non-intubated VATS and VATS in general anesthesia in low-risk patients. Recently some interesting data are emerging (24) with regard to the category of high risk patients (poor respiratory conditions); this study analyzed a total of 293 patients (92 patients non-intubated VATS lung nodule resection) that were offered an awake VATS. The cumulative postoperative morbidity rate was 4.3% which compares favorably with the overall incidence of major respiratory complications in patients undergoing a general anesthesia VATS.
So far, no study has been comparing the occurrence of intraoperative complications between non-intubated VATS and VATS in general anesthesia. Nevertheless, in an analysis of most relevant awake VATS studies summing up a total of 1,441 patients, the overall conversion rate was 2.4%, with an expected discrepancy between minor/intermediate procedures (1%) and major procedures (10%) (55).
Discussion: pros and cons
The proposed advantages of nonintubated VATS (Table 4) include the avoidance of trauma to the hypopharynx, esophagus and trachea (with their potential injuries) from double-lumen endobronchial tube positioning; mechanical ventilator-induced lung injury; the effects of residual neuromuscular block and general anesthetic and analgesic agents (58). Other potential complications of VATS with general anesthesia and double lumen tube intubation include compromised cardiac performance (haemodynamic instability, arrhythmias) and deteriorated early post-operative respiratory function due to a residual diaphragmatic paralysis. In addition, postoperative pain, nausea and vomiting related to anesthetics and opioids, and, the inability to cough leading to respiratory distress (atelectasis) and increasing the risk of pneumonia, represent all possible causes of significant morbidity.

Full table
With nonintubated VATS, patients can ventilate spontaneously, with a more efficient diaphragmatic contraction that, along with lateral decubitus positioning, results in optimal physiological ventilation perfusion matching to the dependent lung. This leads to lower morbidity, faster recovery times, reduced costs and length of hospital stay (62). Hypothetical advantages include an improved respiratory function in the early postoperative period, reduced need for intensive care unit stay, and decreased perioperative morbidity and mortality than with the similar procedures performed using general anesthesia/intubated VATS.
Liu et al. (26) recently have published the largest randomized trial comparing nonintubated VATS with VATS in general anesthesia. They enrolled 354 patients with different thoracic diseases and found that the nonintubated VATS group had a statistically significant decrease in postoperative morbidity rate, most markedly a reduction in post-operative respiratory complications.
Conclusions
Reportedly, awake VATS resection of lung nodules is well-tolerated. The areas for improvement include provision of written information dedicating more time for discussion of the anesthetic modality and the enhancement of post-discharge support. The choice of the anesthetic technique (awake, different grades of sedation with BIS, MAC with a laryngeal mask) must be related to the anesthesiologist’s expertise and experience. The indications, advantages and limitations of awake VATS resection of pulmonary nodules still need to be clarified (Table 5) but, in the hands of experienced surgeons, this procedure appears to be the best technique in elderly patients or those with poor pulmonary function and at high risk for intubation. We expect that, in a relatively short time, available data will significantly increase in order to collect more effective evidence about the real advantages and limitations of nonintubated VATS lung resections concerning postoperative morbidities and mortality, oncological value and procedure-related costs.

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tommaso Claudio Mineo and Marcello Migliore) for the series “Non-intubated Thoracic Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.01.01). The series “Non-intubated Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. GR serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenblatt MB. Pulmonary tuberculosis: evolution of modern therapy. Bull N Y Acad Med 1973;49:163-96. [PubMed]
- Loddenkemper R, Mathur PN, Lee P, et al. History and clinical use of thoracoscopy/pleuroscopy in respiratory medicine. Breathe 2011;8:144-55. [Crossref]
- Rusch VW, Mountain C. Thoracoscopy under regional anesthesia for the diagnosis and management of pleural disease. Am J Surg 1987;154:274-8. [Crossref] [PubMed]
- Elia S, Guggino G, Mineo D, et al. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg 2005;28:312-7. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortications for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Katlic MR, Facktor MA. Non-intubated video-assisted thoracic surgery in patients aged 80 years and older. Ann Transl Med 2015;3:101. [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230-5. [Crossref] [PubMed]
- Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg 1998;65:924-6. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Li J, Liu H, Liu J, et al. Challenges in complex video-assisted thoracoscopic surgery and spontaneous respiration video-assisted thoracoscopic surgery procedures. J Vis Surg 2017;3:31. [Crossref] [PubMed]
- Huang J, Qiu Y, Chen L, et al. Nonintubated spontaneous respiration anesthesia for tracheal glomus tumor. Ann Thorac Surg 2017;104:e161-3. [Crossref] [PubMed]
- Hung WT, Liao HC, Cheng YJ, et al. Nonintubated thoracoscopic pneumonectomy for bullous emphysema. Ann Thorac Surg 2016;102:e353-5. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Lesser TG. Laser application enables awake thoracoscopic resection of pulmonary nodules with minimal access. Surg Endosc 2012;26:1181-6. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Non intubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery--extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Korasidis S, et al. Non intubated thoracoscopic pulmonary nodule resection under spontaneous breathing anesthesia with laryngeal mask. Innovations 2014;9:276-80. [PubMed]
- Liu J, Cui F, Li S, et al. Non intubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]
- Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254-61. [Crossref] [PubMed]
- Wang ML, Galvez C, Chen JS, et al. Nonintubated single-incision video-assisted thoracic surgery: a twocenter cohort of 188 patients. J Thorac Dis 2017;9:2587-98. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [PubMed]
- Yang SM, Wang ML, Hung MH, et al. Tubeless uniportal thoracoscopic wedge resection for peripheral lung nodules. Ann Thorac Surg 2017;103:462-8. [Crossref] [PubMed]
- Piccioni F, Langer M, Fumagalli L, et al. Thoracic paravertebral anaesthesia for awake video-assisted thoracoscopic surgery daily. Anaesthesia 2010;65:1221-4. [Crossref] [PubMed]
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg. 2010;89:1625-7. [Crossref] [PubMed]
- Rocco G. Non-intubated uniportal lung surgery. Eur J Cardiothorac Surg 2016;49:i3-5. [Crossref] [PubMed]
- Irons JF, Miles LF, Joshi KR, et al. Intubated Versus Nonintubated General Anesthesia for Video-Assisted Thoracoscopic Surgery- A Case-Control Study. J Cardiothorac Vasc Anesth 2017;31:411-7. [Crossref] [PubMed]
- American Society of Anesthesiologists (ASA). Distinguishing monitored anesthesia care (MAC) from moderate sedation/ analgesia (conscious sedation). Available online: www.asahq.org
- Gonzalez-Rivas D, Bonme C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Yan TD, Cao C, D’Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-9. [Crossref] [PubMed]
- Beydon L, Rouxel A, Camut N, et al. Sedative premedication before surgery--A multicentre randomized study versus placebo. Anaesth Crit Care Pain Med 2015;34:165-71. [Crossref] [PubMed]
- Wang ML, Hung MH, Chan KC, et al. Intraoperative multiple intercostal nerve blocks exert anesthetic-sparing effect: a retrospective study on the effect-site concentration of propofol infusion in nonintubated thoracoscopic surgery. Acta Anaesthesiol Taiwan 2016;54:77-80. [Crossref] [PubMed]
- Sancheti MS, Dewan BP, Pickens A, et al. Thoracoscopy without lung isolation utilizing single lumen endotracheal tube intubation and carbon dioxide insufflations. Ann Thorac Surg 2013;96:439-44. [Crossref] [PubMed]
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126-30. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostals nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [Crossref] [PubMed]
- Ng CS, Hui JW, Wong RH. Minimizing single-port access in video-assisted wedge resection, with a hookwire. Asian Cardiovasc Thorac Ann 2013;21:114-5. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Moon SW, Cho DG, Cho KD, et al. Fluoroscopy-assisted thoracoscopic resection for small intrapulmonary lesions after preoperative computed tomography-guided localization using fragmented platinum microcoils. Thorac Cardiovasc Surg 2012;60:413-8. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Passera E, Rocco G. From full thoracotomy to uniportal video-assisted thoracic surgery: lessons learned. J Vis Surg 2017;3:36. [Crossref] [PubMed]
- Nan YY, Chu Y, Wu YC, et al. Subxiphoid video-assisted thoracoscopic surgery versus standard video-assisted thoracoscopic surgery for anatomic pulmonary lobectomy. J Surg Res 2016;200:324-31. [Crossref] [PubMed]
- Mineo TC, Tacconi F. From "awake" to "monitored anesthesia care" thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Sinclair SE, Kregenow DA, Lamm WJ, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator induced lung injury. Am J Respir Crit Care Med 2002;166:403-8. [Crossref] [PubMed]
- Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a "triple low" of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology 2012;116:1195-203. [Crossref] [PubMed]
- Hausman MS Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg 2015;120:1405-12. [Crossref] [PubMed]
- Grichnik KP, Clark JA. Pathophysiology and management of one-lung ventilation. Thorac Surg Clin 2005;15:85-103. [Crossref] [PubMed]
- Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 2013;26:40-6. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Vanni G, et al. Immunological and Inflammatory Impact of Non-Intubated Lung Metastasectomy. Int J Mol Sci 2017;18:E1466 [Crossref] [PubMed]
- Mineo TC, Tacconi F. Nonintubated videothoracoscopic operations in thoracic oncology. J Surg 2014;10:22-30.
Cite this article as: Passera E, Rocco G. Awake video-assisted thoracic surgery resection of lung nodules. Video-assist Thorac Surg 2018;3:3.


