
Complex, high-risk thoracic surgery—does risk always outweigh the benefit or can we manage it safely?
Introduction
Thoracic surgical patients often have multiple comorbidities due to the close association between lung cancer, smoking, emphysema and cardiovascular disease. These often predispose them to numerous potentially significant complications. The decision to operate on these patients requires careful assessment of the potential risks and benefits. This is often undertaken as part of a multidisciplinary decision between the patient, respiratory physician, thoracic surgeon and anaesthetist and will endeavour to take into account the extent of the surgery, the surgical approach and comorbid condition.
Despite significant improvements in mortality and morbidity following lung resection over recent years, the risk is not insignificant. In a recent analysis from the European Society of Thoracic Surgeons (ESTS) database looking at almost 48,000 patients, the average 30-day mortality was 2.7% and morbidity was 18.4% (1). The extent of the surgery was found to be a contributing factor, with pneumonectomy carrying an increased mortality at 6.8%, lobectomy at 2.3% and segmentectomy at 1.4%. The most frequent cause of death following lung resection surgery is acute lung injury and acute respiratory distress syndrome, followed by bronchopleural fistula and empyema, cardiac events and cerebrovascular accidents.
Pre-operative assessment of thoracic patients
Surgery is considered to be the best treatment for patients with early stage lung cancer (2), however, in patients with multiple comorbidities this can often lead to impairment of the cardiorespiratory system with potentially serious and life-threatening complications. Patients deemed high-risk have been shown to have a higher incidence of both minor and major cardiopulmonary complications and a longer hospital stay (3).
A number of independent risk factors have been identified in the development of complications in several studies and society databases. These include age, male sex, smoking, chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), cardiac disease, poor lung function with forced expiratory volume in one second (FEV1) less than 60% and carbon monoxide diffusion capacity (DLCO) less than 60%.
Various assessment algorithms and studies to evaluate the risk have been developed, which have been recently summarised to propose an algorithm for daily clinical practice (4).
Firstly, cardiology assessment is paramount in these patients and optimisation by medical, interventional or surgical therapy should be considered prior to further assessment of pulmonary disease. The thoracic revised cardiac risk index (ThRCRI) was derived from lung resection patients by Brunelli et al. in 2010 and looks at four different factors [coronary artery disease (CAD), cerebrovascular disease, serum creatinine level and pneumonectomy]. If the ThRCRI score is equal or more than 2, formal cardiology assessment should be sought for optimisation (5).
Preoperative pulmonary function tests (PFTs) must be performed in all thoracic patients, and if FEV1 is less than 60% the patient should be considered higher risk and evaluated by further functional tests. FEV1 has, however, recently been questioned as an independent factor in assessing risk, and patients should not be excluded from an operation based solely on this measurement. In contrast, the DLCO measurement seems to maintain its ability to evaluate risk as an independent variable and a level of less than 60% should indicate a high-risk patient and lead to further investigations (4).
Formal cardiopulmonary exercise testing (CPEX) is currently regarded as the gold-standard for functional assessment and risk scoring in lung resection surgery (6). Patients with maximum oxygen consumption (VO2max) over 15 mL/kg/min are considered low-risk, whereas patients with a VO2max less than 10 mL/kg/min are deemed high-risk and should be assessed further for alternative strategies such as minor resections or non-surgical therapies. Patients with a VO2max of 10–15 mL/kg/min are considered to be moderate risk and should be carefully assessed and pre-optimised prior to surgery. Recently some studies have looked at further parameters in CPEX testing, with support for the use of the slope of the minute ventilation to carbon dioxide output ratio (VE/VCO2) being realised as a further independent parameter in risk assessment with a value above 35 indicating high-risk (4).
Risk scoring systems in thoracic surgery
Various risk-scoring systems have been developed for thoracic surgery. These models are constantly being re-evaluated and improved as our data sets increase and with improvements in thoracic surgical outcome. They are important tools to provide information and assess the risk during preoperative counselling and discussions with the multidisciplinary team (MDT) and patient to inform shared decision-making.
The European Society Objective Score (ESOS) was the first risk model developed from the ESTS database and was published in 2005 (7). It was derived from the data from 1,753 patients and incorporated only two variables, age and predicted postoperative FEV1 (ppoFEV1), to predict in-hospital mortality following lung resection. It was validated in a number of different patient populations and units (8), however, ESOS has been recently shown to consistently underestimate mortality in our more current patient datasets (9).
The thoracoscore was designed to predict in-hospital mortality for a wide range of thoracic surgical procedures. The risk model is based on data collected from the French Society of Thoracic and Cardiovascular Surgery (10). It was published in 2007 and looked at data from 15,183 patients undergoing general thoracic surgical procedures. The overall mortality rate was found to be 2.2% and nine factors were found to be significantly associated with in-hospital mortality. These factors used to develop the scoring model are age, sex, dyspnoea score, American Society of Anaesthesiologists (ASA) score, performance status (PS) class, priority of surgery, diagnosis group, procedure class and comorbid disease [including smoking, cancer, COPD, hypertension, CAD, diabetes mellitus (DM), peripheral vascular disease (PVD), obesity and alcoholism]. Thoracoscore has been validated in a number of studies (10-12) and is widely adopted nationally and internationally to predict the risk of operative mortality, including use by the British Thoracic Society and National Institute for Clinical Excellence guidelines (13,14). The model has been designed to be used on a wide number of thoracic procedures from minor to invasive operations. A number of independent studies, however, have questioned and criticised its ability to accurately predict the risk in certain subsets of procedures, in particular lung resection surgery and pneumonectomy for lung cancer, and its role to assess an individual patient’s fitness for surgery (15-18).
Eurolung 1 and 2 are the current recommended ESTS risk scoring systems published in 2017 using more recent data from the database (1). 47,960 lung resections between 2007 and 2015 were used for analysis. Cardiopulmonary morbidity was calculated at 18.4% and modelled in Eurolung 1 using a number of variables, including comorbidities and surgical extent and technique, with proportionally weighted points to calculate a score. These scores were then grouped into different classes of incremental morbidity risk. A similar model was produced, Eurolung 2, to predict 30-day mortality. Tables 1,2 show the Eurolung 1 and 2 scoring systems. Both models have been validated and shown to have high precision in predicting the observed morbidity and mortality (9).
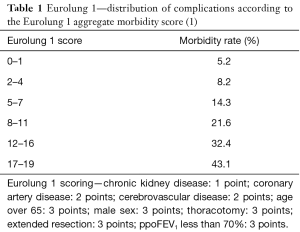
Full table
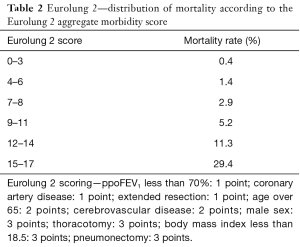
Full table
In addition to the above, a large number of alternative scoring systems have also been developed by different groups and national registries in an attempt to improve on the accuracy of predicting outcomes following thoracic surgery (19-22).
While risk prediction models can provide important information to help inform both the MDTs and patients in making an informed decision regarding the suitability of an operation. It is important to understand the limitations of these models, the patient populations in which these models have been derived and the effect of unmeasured variables and their contribution to outcome. In particular frailty is potentially an important factor which has a great lack of data and evidence surrounding its impact on outcome in thoracic surgery (23). There is also a lack of data and risk scoring for the high-risk procedures such as pneumonectomy, sleeve resections and chest wall resections, and as with all cases, clinical judgement is especially important with these patients.
Scoring systems are important and useful when comparing clinical outcomes between surgeons, units and national and international practices, however, cautions should be applied when interpreting the prediction of morbidity and mortality in an individual patient and assessment of their fitness for surgery, especially in higher risk procedures.
Techniques to lower risk
Video-assisted thoracic surgery
Advances in diagnostic procedures and surgical techniques have improved outcomes in patients with multiple comorbidities, but consequently increasingly complex surgical procedures are being carried out on sicker and older patients. The adoption of video-assisted thoracoscopic surgery (VATS) lung cancer resection has been shown to reduce morbidity and mortality, shorten hospital length of stay and provide at least the same long-term prognosis compared with thoracotomy (2). It is currently the recommended treatment for early stage lung cancer patients and is especially favourable in those higher risk patients, with a number of studies showing a benefit of VATS over thoracotomy in patients with respiratory disease (24,25). This is still a developing technique, and uptake varies geographically. However, the Society of Cardiothoracic Surgeons (SCTS) in the UK shows a significant increase in lung cancer resection performed by VATS over recent years from 23% in 2012–2013 to 30% in 2013–2014 and 40% in 2014–2015 and a reduction in mortality of 0.7% in VATS lobectomy compared with 2% in open lobectomy in 2013–2014 (26).
Enhanced recovery and pre-optimisation
Perioperative management of thoracic surgical patients should aim to focus on techniques that promote early mobilisation and discharge. Key factors to assist in achieving this include the adoption of enhanced recovery programs. This includes a number of perioperative interventions—optimisation of lung function through physiotherapy and treatment of exacerbations, infections or effusions; smoking cessation, improving anaemia and nutritional status; thromboprophylaxis; limiting intraoperative lung injury due to barotrauma or excessive fluid; prompt return to spontaneous ventilation and avoiding post-operative positive pressure ventilation; and the use of appropriate intra and post-operative analgesic techniques incorporating regional or local anaesthetic nerve blocks to allow coughing and post-operative physiotherapy (27).
Multidisciplinary thoracic team approach
The use of high-risk MDT meetings for those patients deemed high-risk for surgical therapy is beneficial to discuss and review pertinent investigations, allow optimisation pre-operatively, refer for specialist input, estimate risk and discuss treatment strategy. Good communication among the MDT, patient and family is important and an understanding of the patient’s quality of life and predicted quality of life can be assessed. A recent paper from the UK suggests that patients involved in a high-risk MDT had the same outcome in terms of complications and mortality as their lower risk counterparts (28). This enabled more patients to be offered a radical treatment option, who might have been refused surgery previously due to their higher risk profile, and provided a robust governance method for decision making and planning in these difficult cases.
Cardiac disease
Cardiovascular complications occur in up to 30% patients following thoracic surgery (29). It is important to identify those at risk and attempt to minimise this prior to surgery.
Arrhythmias
Atrial fibrillation (AF) is the commonest arrhythmia following lung resection. The incidence of AF ranges from 12% to 44%. Patients who develop AF are more likely to develop other complications and it is associated with a longer hospital stay and higher mortality. Risk factors for the development of AF include male sex, older age, extent of surgery, pre-existing cardiac disease and AF, length of procedure and pericardial involvement. In 1/3 of the patients who develop AF the onset is associated with chest infection or sepsis. The Society of Thoracic Surgeons (STS) has published recommendations on the prophylaxis and treatment of AF related to general thoracic surgery. Due to the high morbidity and mortality, prophylaxis should be considered in higher risk patients (30).
Myocardial ischaemia
Perioperative myocardial ischaemia and infarction, a commonly feared complication, is rarely reported with a rate less than 5%, however the mortality can be as high as 40% (29). Thoracic surgical patients often have concomitant cardiac risk factors or overt cardiac disease and rigorous pre-operative assessment and selection can limit the risk. The American College of Cardiology and American Heart Association guidelines for perioperative evaluation and management for non-cardiac surgery is a useful resource; thoracic surgery being classified as high-risk in the context of that guidance (31). Coronary angiography and echocardiography should be performed in the presence of major clinical predictors. In patients requiring pre-operative stenting, surgery should be postponed for 2–4 weeks. Antiplatelet therapy should be continued if possible if within 6 weeks of placement of bare metal stent or within 6–12 months of a drug eluting stent. The continuation of antiplatelet medication, however, needs to be a balance of risk and benefit and involves surgical and cardiology discussion. Patients who are considered at risk of myocardial ischaemia should ideally be invasively monitored intra-and post-operatively, with consideration to intraoperative transesophageal echocardiogram (TOE). Appropriate measures should be taken to maintain cardiac filling pressures and arterial blood pressure at optimum levels and consideration given to the use of inotropes, vasopressors or vasodilators to prevent or limit the sequelae of ischaemia myocardial dysfunction.
Specific high-risk procedures and circumstances
There are a number of specific high-risk procedures and interventions that we will discuss in more detail. The procedures discussed below, however, are highly specialised and require meticulous perioperative planning involving a number of different specialist groups. They should be reserved for the experienced multidisciplinary thoracic team.
Thoracic surgery and extra corporeal membrane oxygenation
The resection of tracheo-bronchial lesions poses a great surgical and anaesthetic challenge (32). The insult to the integrity of the airway requires the anaesthetist to secure the remaining bronchial segments with cross-field ventilation to achieve effective and adequate oxygenation. Whether this is achieved by jet ventilation or instrumentation and securing of the airway across the surgical field, it brings additional difficulty with surgical access and reduced visibility. Both techniques offer a narrow margin of safety and difficulty in further manipulation of the airway in the case of unexpected endobronchial tube displacement, tube cuff leakage or ineffective ventilation during one lung ventilation (33).
To address these difficulties and to ensure effective oxygenation and haemodynamic stability, the use of extra-circulatory support such as veno-arterial extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) has been used. However, these techniques require some degree of anticoagulation and introduce the risk of tumoral cells spreading within the CPB circuit. This together with the complications associated with arterial cannulation can out-weigh the benefit when compared with conventional or cross-field airway management (34,35).
Alternatively, experience with veno-venous ECMO (VV-ECMO) for critical ill patients has increased exponentially after the N1H1 epidemic in 2009 (36). The development of low-resistance and heparin-coated polymethylpentene gas exchange circuits, and the use of percutaneous peripheral venous cannulation (i.e., femoral vein to jugular vein or femoral vein to femoral vein) makes VV-ECMO the extracorporeal life support of choice for complex thoracic surgery (37). To avoid anticoagulation, the drainage and return cannula can be flushed with normal saline until the ECMO pump achieves maximum flow (i.e., between 3.5–4 litre/min). For the majority of patients the ECMO flow is enough to provide effective oxygenation, and the mechanical ventilation can be stopped completely to facilitate the tracheo-bronchial resection. Larger patients, however, may require partial ventilation of the remaining lung parenchyma. Redwan et al. reported a case series of eight patients with a maximum apneic oxygenation of 45 min with normal carbon dioxide concentration (38).
In our Centre, we perform tracheal resections and repairs with the support of VV-ECMO (Figures 1-6). In one case, an apnoeic period of more than 4 h was achieved, until the anastomosis was complete and the patient was allowed to recover spontaneously ventilating through a supraglottic airway device (Figure 7) (39). This enabled avoidance of any potentially harmful positive pressure ventilation after the tracheal reconstruction.
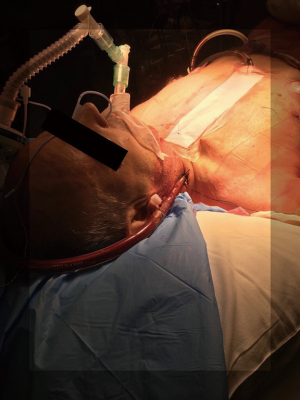
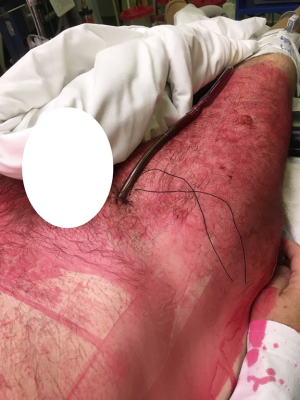
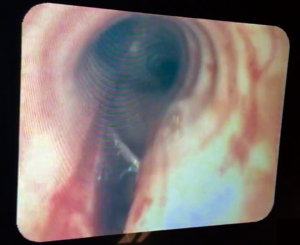
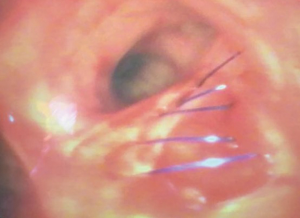
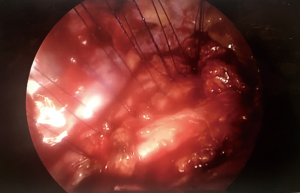

Although ECMO is usually weaned after the tracheo-bronchial tree is restored, it can also be kept early postoperatively to minimize the use of high positive airway pressure or until the patient achieves effective spontaneous ventilation.
Robust planning and close co-operation between the thoracic surgeons and anaesthetist is essential for any thoracic surgery cases, but a collaborative and multidisciplinary approach to the use of ECMO in elective, complex and uncommon thoracic procedures is paramount.
Thoracic surgery and pulmonary hypertension (PH)
Patients with PH have a higher risk of perioperative death than non-PH patients, with right ventricular (RV) dysfunction as the main cause of death (40). The need for one-lung ventilation, the rise in carbon dioxide and the mechanical compression of the pulmonary artery (PA) during thoracic surgery will generally lead to an acute increase in pulmonary vascular resistance (PVR). This is well tolerated in the majority of patients, however, for those with underlying PH, a sudden rise in PVR can trigger a severe RV dysfunction and haemodynamic instability (41).
PH is a haemodynamic state defined as a mean PA pressure of 25 mmHg or above at rest measured by right heart catheterization. The World Health Organization (WHO) classifies PH into five categories, four are pre-capillary and one post-capillary associated with left-sided heart disease (42). PH associated with COPD and chronic thromboembolic diseases are particularly relevant in the thoracic population, however, improvements in medical therapy available for type I (idiopathic PA hypertension) and improved survival, means that this group are also becoming increasingly demanding when presenting for non-cardiac surgery (mean age of diagnosis 50 ± 14 years old) (43).
Understanding the pathophysiology of the different classifications of PH and having a recent echocardiogram to assess the RV are very important in these patients. A recent right heart catheterization to optimise vasodilatory therapy perioperatively is also essential. Type I PH patients are usually on a combination of endothelin receptor antagonists, prostacyclins and/or phosphodiesterase-3 and -5 inhibitors (PDE3 and 5), which must be continued and, where possible, optimised preoperatively.
For patients with chronic thromboembolic PH (type 4), it is essential to maintain anticoagulation for as long as possible prior to the operation and consider full thromboembolic prophylaxis through-out the case, including low molecular weight heparin (LMWH), stockings and intermittent pneumatic compression boots.
The use of PA catheters and TOE should be considered to monitor the RV filling pressures and systolic function during the changes in PVR (i.e., hypoxia, hypercarbia, acidosis and PA clamping) (44,45). The use of vasoconstrictors and inotropic support (i.e., dobutamine, adrenaline) may be required to maintain the RV perfusion pressure and avoid myocardial ischaemia. Regional anaesthetic techniques such as thoracic epidural cause sympathetic block and can have a profound detrimental effect on the homeometric regulation of the RV, reducing its adaptation to a rising afterload (46).
The PH specialist, the thoracic anaesthetist with experience in PH and the intensivist must work in close collaboration with the thoracic surgeon to ensure an appropriate perioperative care plan for these high-risk patients. The multidisciplinary management should aim to maximise pre-operative optimisation and minimise the risk of RV dysfunction perioperatively.
Thoracic surgery and high flow nasal oxygenation
Airway management and oxygenation remains the mainstay of thoracic anaesthesia and the treatment and prevention of hypoxaemic respiratory failure during and post thoracic surgery. Recently, new non-invasive devices have been developed to deliver heated and humidified gas at a very high flow (up to 70 L/min) and at constant oxygen concentrations (from 21% to 100%) (47). Moreover, a number of beneficial physiological effects are well documented, with these devices providing a washout of pharyngeal dead-space, reduction in airway resistance, increase in end-expiratory lung volume and continuous positive airway pressure (CPAP), with a positive linear relationship between gas flow and airway pressure (47,48).
High-flow nasal oxygen (HFNO) is well tolerated and has been increasingly used as an alternative to non-invasive ventilation (NIV) in patients with acute respiratory failure (48). More recently, the use of HFNO has been described in a number of different settings, including pre-oxygenation before intubation (49), post-extubation in the intensive care (50,51), in the emergency department (52) and in patients with heart failure (53). These devices and techniques have also been utilised in thoracic surgery both intraoperatively during flexible bronchoscopy (54,55) and post-operatively to improve and prevent respiratory complications (56).
Bronchoscopy and interventional procedures
Lucangelo and Simon have both studied HFNO in the setting of awake, hypoxaemic patients undergoing flexible bronchoscopy and have demonstrated HFNO can be used to aid oxygenation during these procedures (54,55).
The recent THRIVE study showed that the use of HFNO extended the apnoeic times in patients undergoing general anaesthesia for hypopharyngeal or laryngotracheal surgery (49). Patients were initially pre-oxygenated using HFNO and continued post induction with patency of the upper airway maintained by jaw thrust until a definitive airway was secured. The median apnoea time was 14 min, with a maximum of 65 min. No patient experienced desaturation below 90% and the mean post apnoea carbon dioxide level was 7.8 kPa. This concept of apnoeic oxygenation with CPAP and gas exchange via dead space flushing is an exciting new concept in anaesthesia and has the potential to transform the practice of anaesthesia in emergency and difficult intubations. We feel, however, this could also transform our ability to manage high-risk patients during rigid bronchoscopy and airway intervention and surgery.
In our centre, this concept of apnoeic oxygenation using HFNO has been applied during rigid bronchoscopy (Figures 8,9). It can be a useful aid during tracheal stenting and tracheal tumour debulking in high-risk patients. We have experience with apnoeic oxygenation for more than 30 min in an anaesthetised and paralysed patients to allow the surgeon to access the airway without interference. Ventilation can be resumed at any point during the procedure if required, however, in our limited experience patients remain hemodynamically stable without significant desaturation or hypercarbia.

Post-operatively
As discussed earlier, pulmonary complications are a major cause of morbidity and mortality following thoracic surgery with ALI and ARDS the leading cause of death. Although the evidence for use of HFNO following thoracic surgery is scarce, a recent paper has shown that hospital length of stay following lung resection surgery was reduced in patients receiving HFNO compared to conventional oxygen therapy, despite no difference in the 6-min walk test and spirometry. These patients felt subjectively better and less short of breath and were able to mobilize quicker and go home earlier (56).
Further larger multicentre trials are required to realise the full potential of HFNO and its use in thoracic surgery, but it appears to be an innovative and exciting new therapy with the potential to enhance thoracic surgical practice and improve outcomes.
Awake and non-intubated thoracic surgery
The development of a non-intubated anaesthetic technique during which thoracic surgical patients are operated on while spontaneously ventilating is becoming increasingly popular in some thoracic units (57). These patients are awake or under varying levels of sedation with the aid of regional or local anaesthetic techniques without the use of intubation or positive pressure one-lung ventilation. The concept is to create an iatrogenic pneumothorax as the surgeon enters the chest which can provide excellent lung isolation and operating conditions.
This technique aims to reduce complications from intubation and positive pressure ventilation such as ventilation-induced lung injuries and may be beneficial in patients with severe pulmonary comorbidities in the prevention of post-operative respiratory failure. Awake and minimal sedation techniques also avoid the need for general anaesthesia and thus maintain a more physiological cardiopulmonary and neurological status, which may be beneficial in the high-risk thoracic patient with multiple comorbidities.
Two recent meta-analyses have shown this technique to be safe and to reduce operative morbidity and hospital stay when compared to equivalent procedures performed under GA with intubation and one-lung positive pressure ventilation (58,59). More research is, however, required to understand its benefits in the higher risk populations and patients with particular comorbidities but it remains an exciting field of growing experience and one that may benefit the patient deemed “inoperable”.
Conclusions
With improvements in surgical techniques and anaesthetic management, the definition of high-risk in thoracic surgery is something of a moving target. There are several risk scoring systems in place which use a number of objective criteria to help with preoperative decision making with regards to suitability for a surgical treatment. These scoring techniques, however, fail to incorporate subjective criteria such as the individual patient’s ability, motivation and expectations, the surgical ability and the anaesthetic techniques. It is also clear from the literature, that there is no agreed consensus on the definition of “high-risk” and what this means in terms of outcome.
Surgical resection is the gold-standard treatment for early lung cancer and the decision to operate depends on a number of variables including careful assessment of the potential risks and benefits which will be derived from series and trials in the literature, scoring systems, registry data and the personal experience of the team. It should involve a multidisciplinary approach including shared decision-making with the patient.
New techniques and advances mean that we are able to operate safely on the “high-risk” patient with multiple comorbidities. But this should involve an experienced and dedicated thoracic service with experienced surgeons and anaesthetists, dedicated high-dependency units and ward facilities, and thoracic surgery support staff including nurses, physiotherapists, education teams, and involvement of specialised services (e.g., cardiology, PH team, ECMO) where required. High-risk thoracic surgery requires a truly individualised patient-centred and multidisciplinary approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci and Roberto Crisci) for the series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.09.03). The series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brunelli A, Salati M, Rocco G, et al. European risk models for morbidity (EuroLung1) and mortality (EuroLung2) to predict outcome following anatomic lung resections: an analysis from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2017;51:490-7. [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Sandri A, Papagiannopoulos K, Milton R, et al. High-risk patients and postoperative complications following video-assisted thoracic surgery lobectomy: a case-matched comparison with lower-risk counterparts. Interact Cardiovasc Thorac Surg 2015;21:761-5. [Crossref] [PubMed]
- Salati M, Brunelli A. Risk Stratification in Lung Resection. Curr Surg Rep 2016;4:37. [Crossref] [PubMed]
- Brunelli A, Varela G, Salati M, et al. Recalibration of the revised cardiac risk index in lung resection candidates. Ann Thorac Surg 2010;90:199-203. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S.
- Berrisford R, Brunelli A, Rocco G, et al. The European Thoracic Surgery Database project: modelling the risk of in-hospital death following lung resection. Eur J Cardiothorac Surg 2005;28:306-11. [Crossref] [PubMed]
- Brunelli A, Varela G, Van Schil P, et al. Multicentric analysis of performance after major lung resections by using the European Society Objective Score (ESOS). Eur J Cardiothorac Surg 2008;33:284-8. [Crossref] [PubMed]
- Brunelli A. European Society of Thoracic Surgeons Risk Scores. Thorac Surg Clin 2017;27:297-302. [Crossref] [PubMed]
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32. [Crossref] [PubMed]
- Chamogeorgakis TP, Connery CP, Bhora F, et al. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg 2007;134:883-7. [Crossref] [PubMed]
- Chamogeorgakis T, Toumpoulis I, Tomos P, et al. External validation of the modified Thoracoscore in a new thoracic surgery program: prediction of in-hospital mortality. Interact Cardiovasc Thorac Surg 2009;9:463-6. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- National Institute for Clinical Excellence. Lung cancer: diagnosis and management (CG 121). Published April 2011.
- Sharkey A, Ariyaratnam P, Anikin V, et al. Thoracoscore and European society objective score fail to predict mortality in the UK. World J Oncol 2015;6:270-5. [Crossref]
- Qadri SS, Jarvis M, Ariyaratnam P, et al. Could Thoracoscore predict postoperative mortality in patients undergoing pneumonectomy? Eur J Cardiothorac Surg 2014;45:864-9. [Crossref] [PubMed]
- Bradley A, Marshall A, Abdelaziz M, et al. Thoracoscore fails to predict complications following elective lung resection. Eur Respir J 2012;40:1496-501. [Crossref] [PubMed]
- Barua A, Handagala SD, Socci L, et al. Accuracy of two scoring systems for risk stratification in thoracic surgery. Interact Cardiovasc Thorac Surg 2012;14:556-9. [Crossref] [PubMed]
- Kozower BD, Sheng S, O'brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3 [Crossref] [PubMed]
- Bernard A, Rivera C, Pages PB, et al. Risk model of in-hospital mortality after pulmonary resection for cancer: a national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J Thorac Cardiovasc Surg 2011;141:449-58. [Crossref] [PubMed]
- Brunelli A, Fianchini A, Gesuita R, et al. POSSUM scoring system as an instrument of audit in lung resection surgery. Ann Thorac Surg 1999;67:329-31. [Crossref] [PubMed]
- Poullis M, McShane J, Shaw M, et al. Prediction of in-hospital mortality following pulmonary resections: improving on current risk models. Eur J Cardiothorac Surg 2013;44:238-42; discussion 242-3 [Crossref] [PubMed]
- Dunne MJ, Abah U, Scarci M. Frailty assessment in thoracic surgery. Interact Cardiovasc Thorac Surg 2014;18:667-70. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- SCTS Thoracic Surgery Audit Group. The Thoracic Surgery Brief Report: Audit Years 2011-12 to 2013-14. Society for Cardiothoracic Surgeons in Great Britain and Ireland.
- Jones NL, Edmonds L, Ghosh S, et al. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia 2013;68:179-89. [Crossref] [PubMed]
- Nwaejike N, Elbur E, Malagon I, et al. Is there a role for the high-risk multidisciplinary team meeting in thoracic surgery? Interact Cardiovasc Thorac Surg 2016;22:397-400. [Crossref] [PubMed]
- Keshava HB, Boffa DJ. Cardiovascular complications following thoracic surgery. Thorac Surg Clin 2015;25:371-92. [Crossref] [PubMed]
- Fernando HC, Jaklitsch MT, Walsh GL, et al. The Society of Thoracic Surgeons practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: executive summary. Ann Thorac Surg 2011;92:1144-52. [Crossref] [PubMed]
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215-45. [Crossref] [PubMed]
- Lanuti M, Mathisen DJ. Management of complications of tracheal surgery. Chest Surg Clin N Am 2003;13:385-97. [Crossref] [PubMed]
- Schieren M, Böhmer A, Dusse F, et al. New Approaches to Airway Management in Tracheal Resections-A Systematic Review and Meta-analysis. J Cardiothorac Vasc Anesth 2017;31:1351-8. [Crossref] [PubMed]
- Ulicny KS, Schmelzer V, Flege JB, et al. Concomittant cardiac and pulmonary operation: the role of cardiopulmonary bypass. Ann Thorac Surg 1992;54:289-95. [Crossref] [PubMed]
- Wiebe K, Baraki H, Macchiarini P, et al. Extended pulmonary resections of advanced thoracic malignancies with support of cardiopulmonary bypass. Eur J Cardiothorac Surg 2006;29:571-7; discussion 577-8. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Horita K, Itoh T, Furukawa K, et al. Carinal reconstruction under veno-venous bypass using a percutaneous cardiopulmonary bypass system. Thorac Cardiovasc Surg 1996;44:46-9. [Crossref] [PubMed]
- Redwan B, Ziegeler S, Freermann S, et al. Intraoperative veno-venous extracorporeal lung support in thoracic surgery: a single-centre experience. Interact Cardiovasc Thorac Surg 2015;21:766-72. [PubMed]
- Irons JF, Martinez G. Complex, high-risk thoracic surgery—does risk always outweigh the benefit or can we manage it safely? Asvide 2017;4:401. Available online: http://www.asvide.com/articles/1715
- Pilkington SA, Taboada D, Martinez G. Pulmonary hypertension and its management in patients undergoing non-cardiac surgery. Anaesthesia 2015;70:56-70. [Crossref] [PubMed]
- Wilkinson JN, Scanlan M, Skinner H, et al. Right heart function during onelung ventilation: observations using transoesophageal echocardiography. Anaesthesia 2009;64:1387-8. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62:D51-D59. [Crossref] [PubMed]
- Howard LS, Grapsa J, Dawson D, et al. Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev 2012;21:239-48. [Crossref] [PubMed]
- Sullivan B, Puskas F, Fernandez-Bustamente A. Transesophageal echocardiography in noncardiac thoracic surgery. Anesthesiol Clin 2012;30:657-69. [Crossref] [PubMed]
- Missant C, Rex S, Claus P, et al. Thoracic epidural anaesthesia disrupts the protective mechanism of homeometric autoregulation during right ventricular pressure overload by cardiac sympathetic blockade: a randomised controlled animal study. Eur J Anaesthesiol 2011;28:535-43. [Crossref] [PubMed]
- Hernández G, Roca O, Colinas L. High-flow nasal cannula support therapy: new insights and improving performance. Critical Care 2017;21:62. [Crossref] [PubMed]
- Lee JH, Rehder KJ, Williford L, et al. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med 2013;39:247-57. [Crossref] [PubMed]
- Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70:323-9. [Crossref] [PubMed]
- Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA 2016;315:1354-61. [Crossref] [PubMed]
- Hernández G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016;316:1565-74. [Crossref] [PubMed]
- Jones PG, Kamona S, Doran O, et al. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care 2016;61:291-9. [Crossref] [PubMed]
- Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care 2013;28:741-6. [Crossref] [PubMed]
- Lucangelo U, Vassallo FG, Marras E, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract 2012;2012:506382.
- Simon M, Braune S, Frings D, et al. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy-a prospective randomised trial. Critical Care 2014;18:712. [Crossref] [PubMed]
- Ansari BM, Hogan MP, Collier TJ, et al. A randomized controlled trial of high-flow nasal oxygen (Optiflow) as part of an enhanced recovery program after lung resection surgery. Ann Thorac Surg 2016;101:459-64. [Crossref] [PubMed]
- Irons JF, Martinez G. Anaesthetic considerations for non-intubated thoracic surgery. J Vis Surg 2016;2:61. [Crossref]
- Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31-40. [Crossref] [PubMed]
- Tacconi F, Pompeo E. Non-intubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016;8:S364-75. [Crossref] [PubMed]
Cite this article as: Irons JF, Martinez G. Complex, high-risk thoracic surgery—does risk always outweigh the benefit or can we manage it safely? Video-assist Thorac Surg 2017;2:63.


