Non-intubated video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax
Introduction
Non-intubated video-assisted thoracic surgery (VATS) is a synonym for awake thoracic surgery. With the developments in thoracoscopic techniques, awake thoracic surgery is being performed routinely in the recent years. However, little attention has been given to the details of the procedure, intraoperative care, and respiratory conditions of awake thoracic surgery for primary and secondary spontaneous pneumothorax. The purpose of this article was to describe those parameters during awake thoracic surgery.
Preoperative preparation
The three causes of hypoxia during non-intubated VATS (also known as awake VATS or A-VATS) are hypoventilation (low alveolar ventilation), right-to-left shunt, and tachypnea. Our first concern is hypoventilation during A-VATS because of ongoing artificial pneumothorax, but it does not always occur during A-VATS. Generally, during hypoventilation, P(A-a)O2 is normal. PaCO2 is elevated, and increasing the fraction of inspired oxygen can alleviate the hypoxemia; meanwhile, the hypercapnia can be corrected by mechanically ventilating the patient to eliminate CO2. Another cause of hypoventilation can be a disease involving the respiratory muscles (diaphragm, intercostal muscles, etc.) or the nerves supplying the respiratory muscles. Normally, the pressure in the pleural space is negative with reference to the atmospheric pressure. However, during A-VATS, the pressure in the pleural space may be similar to the atmospheric pressure, thus revealing the artificial pneumothorax. The main physiologic consequences of a pneumothorax are decrease in vital capacity and PaCO2. In normal A-VATS conditions, a decrease in vital capacity is almost well tolerated. If a patient’s lung function is compromised because of a severe lung disease occurring before the pneumothorax, then the decrease in vital capacity may lead to respiratory insufficiency with hypoventilation and respiratory acidosis. Thus, simulating such respiratory conditions preoperatively is important in A-VATS. In fact, a chest tube is not connected to an aspiration system under the condition of atmospheric pressure. Then, respiratory and circulatory conditions (blood pressure, heart rate, SPO2, etc.) are assessed approximately 5 minutes after simulating A-VATS conditions. An SPO2 above 93% and unchanged blood pressure and heart rate after 5 minutes are good signs.
Our next concern is the right-to-left shunt. The reduction in PaO2 appears to be due to both anatomic shunts and areas of low ventilation/perfusion ratios in the partially atelectatic lung. Larger pneumothoraces are associated with greater shunts. Pneumothoraces occupying less than 25% of the hemithorax are not associated with increased shunts (1). In fact, although artificial hemopneumothoraces occupy almost less than 25% volume during A-VATS, if the collapse of a lung were to progress, the air in the pleural space can be temporarily evacuated under the closed drainage system.
Our last concerned is tachypnea. The causes of tachypnea are hypoxia, thoracic pain, or mental factors due to the ongoing awake surgical procedure, with mental factor being extremely important. It is not rare to interrupt an awake surgery in view of tachypnea due to anxiety. In such a case, administering highly selective α2-adrenergic receptor agonists (e.g., dexmedetomidine) is useful. The effects of dexmedetomidine on respiration appear to be similar to those of deep sleep. Dexmedetomidine does not suppress the respiratory function, even at high doses.
Control of pain
An epidural catheter was inserted at the T3–T7 level before the non-intubated thoracic surgery. Then, a continuous infusion of 0.2% ropivacaine was started. We used not only epidural anesthesia but also thoracic paravertebral block to achieve adequate surgical anesthesia for the pneumothorax during A-VATS. Postoperative analgesia was basically accomplished by continuous infusion of ropivacaine via the epidural catheter for 3 to 5 days. Additional analgesics consisting of non-steroidal anti-inflammatory drugs and/or opioids were given as needed.
Non-intubated surgical procedure
Ventilation was maintained by spontaneous breathing throughout the operation with oxygen supplementation, maintaining an SpO2 level >93%. Patients were placed in supine or hemi-lateral decubitus position when possible, and local anesthesia with 1% lidocaine was administered; thereafter, a 5-mm port was inserted in the intercostal space at the mid-clavicular line. A 5-mm, 30° end-viewing flexible scope (LTF TYPE 260; Olympus, Tokyo, Japan) was introduced through the 12-mm thoraco-port. When desaturation was noted on the monitor, the lung was temporarily re-expanded by sucking the air in the pleural cavity through the double-lumen chest drainage tube. A second small incision was made in the intercostal space at the anterior axillary line. A Roeder loop was introduced to ligate a ruptured bulla. When the air leak point was not suitable for ligation, the area was stapled using the Endo-GIA stapling device (Tyco Healthcare Japan Inc., Tokyo, Japan) inserted through a third small intercostal incision. The area of ligation or the staple line was additionally reinforced with a non-woven polyglycolic acid sheets (NEOVEIL®; Gunze Co., Ayabe, Japan) and fibrin glue. In all cases, a 20-Fr double-lumen chest tube or 19-Fr flexible drainage tube was inserted through an inferior incision and placed at the thoracic space. Postoperatively, the chest bottles were connected to a suction with pressure of −5 cmH2O until the air leak stopped. For persistent postoperative air leak over 3 days, chemical pleurodesis with OK-432 or 50% glucose solution was performed.
Communication between the anesthesiologist and the surgeon
It is extremely important for a surgeon to work cooperatively with an anesthetist for A-VATS to work. Everyone involved needs to be prepared to convert to general anesthesia during both procedures and should not attempt a regional technique in patients with a suspected difficult airway. However, we have never had the need to convert to general anesthesia during A-VATS and preoperatively transmitted that information to the anesthesiologist. In our A-VATS, a lung is usually re-expanded by pausing the surgical procedure, because intrathoracic air can be aspirated through the drainage tube anytime. Therefore, discontinuing A-VATS is possible at any point should the surgery prove to be difficult. Such cases requiring discontinuation were due to alveolar air leak points with severe intrathoracic adhesion that were not previously detected. We performed saline-filled computed tomographic thoracography to detect pleural fistulae (presenting as bubble signs) in patients with intractable secondary spontaneous pneumothorax (2). The preoperative identification of such air leak points resulted in successful A-VATS. Furthermore, in cases where such air leak points were not identified, we performed chemical pleurodesis using fibrin glue or talc during A-VATS.
Comparison of operative stress between A-VATS and general anesthesia
Vanni et al. (3) reported that A-VATS resulted in a lesser impact on postoperative lymphocyte responses than procedures performed under general anesthesia, as shown by the significant difference in the postoperative proportion of natural-killer cells. In our study, the incidence of postoperative respiratory complications, including pneumonia and acute respiratory distress syndrome, were significantly lower in the A-VATS group than in the general anesthesia group (4).
Cases of awake thoracoscopic procedures
A-VATS for primary spontaneous pneumothorax
Single-port thoracic surgery or reduced-port surgery has recently attracted much attention as one of the minimally invasive thoracic surgeries. Reduced-port thoracic surgery in awake conditions for primary spontaneous pneumothorax is advantageous in terms of cosmetic appearance and pain. Single-port thoracic surgery is operated with instruments inserted from the single extended incision, and reduced-port surgery is operated with instruments punctured without extending incision. Reduced-port surgery for primary spontaneous pneumothorax in awake conditions is less invasive than the conventional three-port VATS under general anesthesia. Hence, reduced-port thoracic surgery in awake conditions is predicted to be more widely used in the future.
Pneumothorax in pregnancy
A 31-year-old female patient in 9th week of gestation complained of chest pain and dyspnea. She was diagnosed to have intractable pneumothorax of pregnancy, but we were undecided between surgery and conservative therapies because of the possible influence of chemical pleurodesis or drug administration under general anesthesia on both the mother and the baby. We obtained informed consent from the patient to perform surgical interventions in awake conditions and performed a minimally invasive surgery using thoracoscopic approach. Based on the results of the procedure, we conclude that awake surgical intervention is applicable in selected patients, such as women with pneumothorax in pregnancy, and is particularly useful in those in whom general anesthesia is best avoided (5).
A-VATS for Gorham-Stout disease
Gorham-Stout disease is an extremely rare disease characterized by proliferation of vascular and lymphatic structures. A 15-year-old male patient complaining of dyspnea and cervical pain presented to a local hospital, and he was diagnosed with Gorham-Stout disease of the cervical spine with chylothorax. To detect, and possibly treat, the lymphatic leakage, we performed classical lymphangiography using ethiodized oil (lipiodol). This procedure confirmed the chylous leakage around the right apical area in the right thoracic space. Because of the progression of cervical dislocation and malnutrition, awake thoracoscopic surgery was performed (6).
Intractable right pneumothorax after left pneumonectomy
A 74-year-old male patient underwent left pneumonectomy. One month later, he developed right pneumothorax, necessitating the placement of a drainage tube. Because of severe air leakage and incomplete re-expansion of the right lung, we abandoned additional pleurodesis and chose VATS with local and epidural anesthesia in view of his poor general medical condition (7).
Bilateral intractable secondary spontaneous pneumothorax
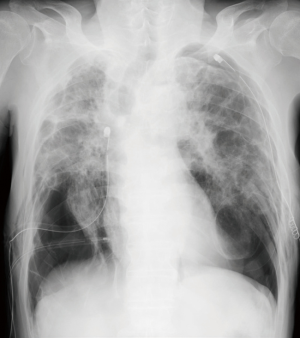
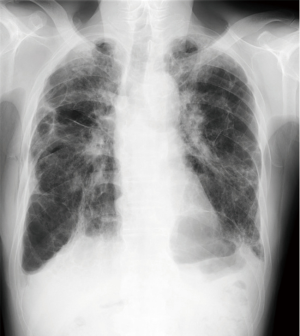
A 74-year-old man presented to the emergency department with sudden onset of severe breathlessness. Chest X-ray showed bilateral pneumothorax. We inserted right and left thoracic drainages (Figure 1), but continuous air leakage was revealed in the left thoracic drainage system. Therefore, awake thoracoscopic surgery was performed on the left lung. Two days postoperatively, the left drainage tube was removed. Subsequently, chemical pleurodesis using diluted fibrin glue was performed on the right lung and then the right drainage tube was removed (Figure 2).
Tips, tricks, and pitfalls
A-VATS is contraindicated in patients with pneumothorax with severe pleural adhesions. Preoperative identification of air leak points is one of the keys to the success of this procedure. A disadvantage of A-VATS is the difficulty in finding air leak points intraoperatively because the use of water-sealing test with positive airway pressure is impossible with this procedure. Notably, severe pleural adhesions, which obstruct satisfactory thoracoscopic view, are common in secondary spontaneous pneumothorax.
Conclusions
The use of non-intubated VATS for primary and secondary spontaneous pneumothorax, performed by skilled thoracic surgeons in cooperation with anesthetists, is a feasible surgical option.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tommaso Claudio Mineo and Marcello Migliore) for the series “Non-intubated Thoracic Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.08.09). The series “Non-intubated Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). We obtained informed consent from the patient to perform surgical interventions in awake conditions and performed a minimally invasive surgery using thoracoscopic approach.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norris RM, Jones JG, Bishop JM. Respiratory gas exchange in patients with spontaneous pneumothorax. Thorax 1968;23:427-33. [Crossref] [PubMed]
- Watanabe T, Noda M, Okazaki T, et al. Preoperative saline-filled computed tomography thoracography for awake video-assisted thoracic surgery: report of three cases. Surg Today 2015;45:1579-82. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Noda M, Okada Y, Maeda S, et al. Is there a benefit of awake thoracoscopic surgery in patients with secondary spontaneous pneumothorax? J Thorac Cardiovasc Surg 2012;143:613-6. [Crossref] [PubMed]
- Onodera K, Noda M, Okada Y, et al. Awake video-thoracoscopic surgery for intractable pneumothorax in pregnancy by using a single portal plus puncture. Interact Cardiovasc Thorac Surg 2013;17:438-40. [Crossref] [PubMed]
- Noda M, Endo C, Hoshikawa Y, et al. Successful management of intractable chylothorax in Gorham-Stout disease by awake thoracoscopic surgery. Gen Thorac Cardiovasc Surg 2013;61:356-8. [Crossref] [PubMed]
- Noda M, Okada Y, Maeda S, et al. Successful thoracoscopic surgery for intractable pneumothorax after pneumonectomy under local and epidural anesthesia. J Thorac Cardiovasc Surg 2011;141:1545-7. [Crossref] [PubMed]
Cite this article as: Noda M. Non-intubated video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax. Video-assist Thorac Surg 2017;2:56.