
Uniportal VATS lymphadenectomy
Introduction
Lymphadenectomy is an intrinsic part of surgery for lung cancer. Over the years there have been discrepancies about the possible curative effect of it, but it is clear that improves accurate staging. One of the fears and criticism in the past over the use of minimally invasive approaches has been the possibility to perform a lymphadenectomy without an open incision. These fears and criticisms could potentially be applied again to the further advance of VATS surgery, the uniportal VATS (uniVATS) approach. Authors have demonstrated in recent literature of the equivalency between the uniVATS and the traditional multiport VATS approaches in respect of lymph nodes access and yield (1-4).
Indeed, when accurate and honest audit and report of the extent of lymphadenectomy during pulmonary surgery even with a thoracotomy approach there are shortcomings even in leading oncological units (5-7). There is now extensive evidence in the form of case series and comparison of cohorts that the approach via uniVATS does not carry any limitations per se, and the limits are defined by experience and dedication to lymphadenectomy (8-10).
The traditional uniVATS approach is performed via a limited anterior incision without the use of a rib spreader with an extension that would permit retrieval of the specimen. We recommend to maintain the optics at the most lateral end of the incision trying to occupy as little of the wound as possible that will allow insertion and manipulation of instruments without crowding. With this approach and the optics at the apex of the pleural cavity in the lateral position, all areas of the pleural cavity can be exposed and visualised (11). We describe the techniques of the different nodal stations
Station 8 and 9 (inferior pulmonary ligament and paraesophageal)
In order to access the lower stations with the uniVATS approach it is recommended to place the optic in the most lateral edge of the incision. It is directed towards the phase of the pleural cavity with the lower lobe retracted cranially to allow exposure of the inferior pulmonary ligament. The optic and the retractor can be hold by the assistant while the surgeon uses bimanual instrumentation to divide the inferior pulmonary ligament. During the initial division of the ligament it is sometimes helpful to push the diaphragm caudally if it is elevated due to the muscle relaxation during anaesthesia and even more in obese patients.
We favour the use of energy devices although electrocautery can be as effective. Once the inferior ligament is divided the posterior mediastinal pleura is divided towards the subcarinal space and stations 8 and 9 are exposed (Figure 1). As with every station, both extensive dissection (lymph nodes and whole fatty perinodal tissue) or an excision directed towards lymph nodes only can be achieved.

Excision of these stations rarely cause bleeding or complications, although care with surrounding structures is important. These are the oesophagus which can be visualised once posterior mediastinal pleura is divided, the inferior pulmonary vein, and the thoracic aorta which can be damaged accidentally if the electrocautery is not well controlled depth-wise.
Station 7 (subcarinal)
The subcarinal space is, in the opinion of the authors, the one that requires more dedication to explore by surgeons both in open and minimally invasive approaches. It is understandable that following a difficult pulmonary resection the surgeon’s enthusiasm to continue the procedure can be impaired. A way to deal with this could be to perform the lymphadenectomy at the start of the procedure, which can also aid later dissection. The uniVATS approach subcarinal exploration before or after the pulmonary resection.
We do favour tilting the operating table towards the surgeon in order to expose the back of the lung easier. By division of the posterior mediastinal pleura upwards from the inferior pulmonary ligament the subcarinal space is identified below the inferior border of the lower bronchus, between the inferior pulmonary vein and the lower bronchus itself. The optic remains at the most lateral edge of the incision, and the lung is retracted anteriorly either by grasping it or by pressure forwards with a pledget (Figures 2,3).


Bimanual instrumentation with either a lymph node grasper and suction or energy device/electrocautery permits progressive excision of station 7 lymph nodes. This station accessed posteriorly must be explore in depth in order to remove the subcarinal space and not only the lymph nodes below the lower bronchus. If required, the contralateral main bronchus can be visualised via uniVATS.
Exploration of the subcarinal space can frequently cause some bleeding from the lymph nodes or their small feeding vessels, but more importantly from the bronchial arteries that can be damaged during excision. We believe that the use of energy devices helps in maintaining haemostasis during the surgery.
The subcarinal space during uniVATS can also be accessed via an “anterior approach” in the right side between the inferior pulmonary and middle lobe veins, and in the left side between the inferior pulmonary and lingular veins, where the lower lobe bronchus can be found and its inferior border followed towards the station 7 lymph nodes.
Stations 5 and 6 (aortopulmonary window and pre-aortic)
In left sided procedures stations 5 and 6 can be accessed, normally at the initial stages of surgeries for upper lobe as they will help dissection and control of the left main pulmonary artery. The mediastinal pleura proximal to the main pulmonary artery is divided and will expose station 5 lymph nodes (Figure 4). By extending the division of the mediastinal pleura over the aortic arch station 6 is identified. Left hilar station 10 is in close proximity and exposed by extending the dissection of the mediastinal pleura anteriorly towards the hilar structures. The optics remain at the most lateral edge of the wound and the upper lobe is retracted caudally although sometimes raising the head of the operating table suffices.

Bimanual instrumentation by the surgeon with the use of grasper forceps and blunt (by suction) or sharp (by energy device or electrocautery) dissection permits excision of these stations (Figure 5). In the uniVATS approach these stations as well as the initial branches of the pulmonary artery are very easily identified and dissected as they are placed directly in front of the optic and instruments. It is clearly very important to maintain control of instruments depth-wise to avoid extremely rare injuries to the aortic adventitia or main pulmonary artery.
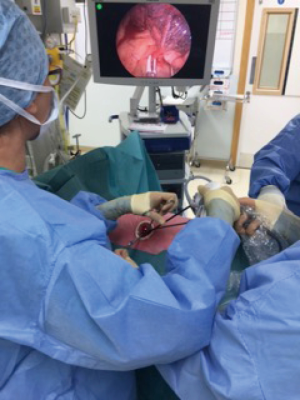
We do recommend in left upper lobe resections to continue the division of the posterior mediastinal pleura caudally in order to facilitate dissection and division of structures later on during the lobectomy.
Station 4L (left lower paratracheal)
During left lung resections, the lower paratracheal space is probably the less explored station both in the open or the VATS approach. It is a station placed deep to the aortopulmonary window, the recurrent laryngeal nerve can be at risk, and in some cases the ligamentum arteriosum can difficult exposure.
Once the station 5 is excised, the base of that space can be explored with care not to damage the left recurrent laryngeal nerve. With care, the station 4L can be reached and excised up to the point of visualising the left side of the trachea (Figure 6). We do favour to perform exploration without the use of electrocautery to avoid neural damage. Potential bleeding from this station is virtually always related to small feeding vessels and can be stopped by pressure or with the use of routine haemostatic agents, not requiring cauterization of this area. In cases of prior extensive cervical mediastinal dissections (VAMLA or TEMLA) this area does not require difficult exploration during lung resection.

Station 4R and 2R (right lower and upper paratracheal)
The right paratracheal area can be explored via uniVATS either by lifting the azygos vein or by division of the paratracheal mediastinal pleura above the azygos (Figure 7). The chain is continued caudally with station 10 (hilar) behind the azygos vein, so this dissection is normally performed at the same time during surgery. The optic remains at the lateral edge of the wound and the upper lobe is retracted caudally. As in the left side, elevation of the head of the operating table or performing the upper lobectomy prior to the lymphadenectomy can avoid the use of the retractor.

The lymph node alongside the extensive fat tissue in this area can be excised with bimanual instrumentation between the right side of the trachea and posterior to the superior vena cava (SVC). These stations can bleed due to sizeable feeding vessels and we recommend the use of energy devices with or without further use of local haemostatics. UniVATS exposure allows very proximal exploration of this paratracheal space to the level of higher lymph nodes.
Station 3 (anterior pretracheal)
During right-sided surgeries, the anterior station 3 can be accessed by incising the pleura medially to the SVC, above the level that the right main pulmonary artery crosses behind the SVC.
Once the space is entered, again bimanual instrumentation will permit the dissection and excisions of the lymph nodes (Figure 8). The optic is directed toward the front of the patient and the table can be rotated towards the back of the patient to improve exposure.

Stations 10L and 10R (hilar)
Dissection of the hilar lymph nodes prior to hilar structures during lobectomy will help without a doubt further progress of the procedure. In the left side involves not only excision of the lymph nodes anterior to the main pulmonary artery and superior vein, but by dividing the posterior mediastinal pleura we can access station 10 lymph nodes posterior to the upper bronchus (Figure 9). Care has to be taken to try to preserve the main vagus nerve, and not to injury the branch of the pulmonary artery to segments 2 and 6. On the right side, this station can extend under the azygos vein and continue cranially into station 4R.

On this side, we should avoid damage to the upper trunk of the pulmonary artery that lies under the station 10, and the azygos vein itself (Figure 10). Once this station is excised, the access to the upper trunk of pulmonary artery and its plane to the upper bronchus becomes very easy to dissect via uniVATS approach.

Summary
UniVATS provides an excellent access to all mediastinal nodal stations during pulmonary resection. The use of lung retraction in addition to movement of the operating table facilitates the procedures. There are no limitations of the technique, rather surgeons’ philosophy and experience. Reports indicate that with uniVATS the yield of mediastinal lymphadenectomy is at least equal as with traditional multiport VATS and to open techniques.
Starting the procedure performing the lymphadenectomy can ensure a more complete exploration and help with subsequent dissection of the hilar structures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci and Roberto Crisci) for the series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.08.16). The series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mu JW, Gao SG, Xue Q, et al. A Matched Comparison Study of Uniportal Versus Triportal Thoracoscopic Lobectomy and Sublobectomy for Early-stage Nonsmall Cell Lung Cancer. Chin Med J (Engl) 2015;128:2731-5. [Crossref] [PubMed]
- Zhu Y, Liang M, Wu W, et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med 2015;3:92. [PubMed]
- Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?. Eur J Cardiothorac Surg 2016;49:i64-72. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Hagan ME, Williams ST, Socci L, et al. Completing the audit cycle improves surgical standards in lung cancer. Why do some patients still not receive the best care? J Thorac Oncol 2013;8:779-82. [Crossref] [PubMed]
- Yang H, Li XD, Lai RC, et al. Complete mediastinal lymph node dissection in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Thorac Cardiovasc Surg 2013;61:116-23. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Thoracoscopic lobectomy through a single incision. Multimed Man Cardiothorac Surg 2012;2012:mms007.
- Martin-Ucar AE, Socci L. Stations 8–9. After division of the inferior pulmonary ligament the posterior mediastinal pleura is divided cranially exposing the regional lymph nodes. Asvide 2017;4:382. Available online: http://www.asvide.com/articles/1696
- Martin-Ucar AE, Socci L. Station 7. From the left side, the lung is retracted anteriorly and table is rotated towards the surgeon. Asvide 2017;4:383. Available online: http://www.asvide.com/articles/1697
- Martin-Ucar AE, Socci L. Station 7. On the right side, the posterior mediastinal pleura is divided, and with the lung retracted anteriorly, the subcarinal area is explored below the right main bronchus. Asvide 2017;4:384. Available online: http://www.asvide.com/articles/1698
- Martin-Ucar AE, Socci L. Station 5. The aortopulmonary window can be explored by dissection of the mediastinal pleura below the aorta. Asvide 2017;4:385. Available online: http://www.asvide.com/articles/1699
- Martin-Ucar AE, Socci L. Station 4L. After excision of station 5 the area below the aortic arch is entered and left paratracheal station can be accessed. Asvide 2017;4:386. Available online: http://www.asvide.com/articles/1700
- Martin-Ucar AE, Socci L. Station 4R and 2R. Exposure of the right paratracheal stations by excising the mediastinal pleura above the azygos vein just lateral to the superior vena cava. Asvide 2017;4:387. Available online: http://www.asvide.com/articles/1701
- Martin-Ucar AE, Socci L. Station 3A. On the right side, the mediastinal pleura medially to the superior vena cava is divided, with exploration of the anterior tracheal area above the level of the right main pulmonary artery. Asvide 2017;4:388. Available online: http://www.asvide.com/articles/1702
- Martin-Ucar AE, Socci L. Station 10L. Excision of a posterior hilar lymph node on the left side. Asvide 2017;4:389. Available online: http://www.asvide.com/articles/1703
- Martin-Ucar AE, Socci L. Station 10R. Excision of a localised hilar lymph node on the right side. Asvide 2017;4:390. Available online: http://www.asvide.com/articles/1704
Cite this article as: Martin-Ucar AE, Socci L. Uniportal VATS lymphadenectomy. Video-assist Thorac Surg 2017;2:55.


