
Nonintubated thoracoscopic anatomical segmentectomy for lung cancer: a single-center experience with consecutive 89 cases
Introduction
Lung cancer is the leading cause of cancer death worldwide (1). Among all lung cancer, more than 80% are non-small cell lung cancer (NSCLC). Although a thoracoscopic lobectomy is considered the best treatment for patients with early-stage NSCLC (2), a less invasive anatomical segmentectomy is being increasingly re-evaluated recently, not only in geriatric patients with impaired cardiopulmonary reserve who cannot tolerate a lobectomy, but also in patients with small peripheral lung cancers 2 cm or smaller (3,4).
Intubated general anesthesia with a double-lumen endobronchial catheter or an endobronchial blocker is the generally accepted standard to facilitate surgical exposure and one-lung ventilation during video-assisted thoracic surgery (VATS) (5). However, complications after general anesthesia and tracheal intubation are not negligible, including intubation-related airway injury, residual neuromuscular blockade, postoperative pulmonary complications, impaired cardiac performance and nausea and vomiting after surgery (6). To overcome the adverse effects of intubated general anesthesia, we developed a nonintubated thoracoscopic technique and reported that it is technically feasible performing nonintubated anatomical segmentectomy for management of lung cancer as well (7-10). The combination of VATS segmentectomy and nonintubated anesthesia can therefore be a less invasive treatment option for lung cancer. In this study, we report our overall experience with this nonintubated thoracoscopic approach for anatomical segmentectomy in patients with NSCLC to further determine the safety and short-term outcomes of nonintubated thoracoscopic anatomical segmentectomy using regional thoracic anesthesia and targeted sedation.
Methods
This study was approved by the Research Ethics Committee of National Taiwan University Hospital (201509053RINC). All patients gave their consent for nonintubated VATS and clinical data collection of potential publication before surgery.
Patients
We began performing nonintubated VATS for surgical management of lung tumors in August 2009. Afterwards, we prospectively maintained a database of all patients undergoing nonintubated VATS. The nonintubated VATS database was then searched retrospectively to identify lung cancer patients who underwent VATS anatomical segmentectomy for this study. Our thoracic surgical team, comprising surgeons and anesthesiologists, selected appropriate patients for nonintubated VATS after reviewing their medical records and results of physical examinations. The criteria for patients undergoing nonintubated thoracoscopic segmentectomy included those with clinical stage I NSCLC with tumors smaller than 2 cm, and those with impaired cardiopulmonary function who were not considered suitable for a lobectomy. Specifically, compromised nonintubated VATS segmentectomy included elderly patients with a New York Heart Association functional classification of II or higher, or patients with a preoperative forced expiratory volume in one second less than 70% predicted (10). Additionally, patients with an American Society of Anesthesiologists score more than 3, a history of bleeding disorder, sleep apnea, previous ipsilateral thoracic surgery, evidence of pleural adhesions, unfavorable airways or spinal anatomy, or chest wall deformity were also excluded.
Anesthetic management of nonintubated VATS
Our anesthesia protocol for nonintubated VATS was previously reported (8-11). In summary, premedication of fentanyl (50 µg) and glycopyrrolate (0.2 mg) were given intravenously. Standard monitoring included electrocardiogram, pulse oximetry, arterial blood pressure, and respiratory rate. We measured end-tidal carbon dioxide (EtCO2) via a nasal detector noninvasively. With full sterile preparation, an epidural catheter was inserted into the T5–6 thoracic interspace to achieve and maintain a sensory block between T2 and T9 dermatomes using 2% lidocaine. Patients were sedated with intravenous propofol infusion using a target-controlled infusion method, aiming a sedation level measured by a bispectral index (BIS) sensor (BIS Quatro, Aspect Medical System, Norwood, MA) applied to the forehead of each patient to obtain a BIS value between 40 and 60. Incremental intravenous injection of fentanyl 25 µg was given to obtain a respiration rate between 12 and 20 breaths/min. The patients were then placed in the lateral decubitus position.
Regional thoracic anesthesia was achieved by thoracic epidural anesthesia between 2009 and 2012. We began to adopt internal intercostal nerve block from March 2012 as an alternative for thoracic epidural anesthesia because it is easier and more time-saving (8,12). For patients undergoing intercostal nerve blockade, a thoracoscopic port was initially created after local infiltration of 2% lidocaine. Intercostal nerve block was then produced under direct thoracoscopic vision by infiltration of 0.5% bupivacaine with a 25-gauge top-winged infusion needle (1.5 mL for each intercostal space) from the third to the eighth intercostal nerve under the parietal pleura, 2 cm laterally to the sympathetic chain (8).
During the procedure, patients breathed oxygen through a ventilation mask, keeping oxygen saturation above 90%. To prevent coughing during thoracoscopic manipulation, an intrathoracic vagal block using 0.5% bupivacaine (2–3 mL) was produced in all patients, at the level of the lower trachea for right-sided procedures and at the level of the aortopulmonary window for left-sided procedures. At the end of surgery, the operated lung was manually expanded via positive-pressure mask ventilation to check the presence of air leaks. After wound closure and chest tube insertion, patients were fully awake and were asked to breathe deeply and cough to re-expand the collapsed lung further.
Technique of thoracoscopic segmentectomy
All patients underwent a complete anatomical segmentectomy with mediastinal lymph node dissection for pathological staging of primary lung cancer. Thoracoscopic segmentectomy was performed using a 3-port method (13). In brief, the patient was placed in the full lateral decubitus position, with slight flexion at the level of the mid-chest. The thoracoscope was placed in the 7th or 8th intercostal space in the mid-axillary line. A working port was placed in the sixth or seventh intercostal space in the auscultatory triangle, and a 3- to 5-cm utility incision was placed anteriorly in the 5th intercostal space.
After collapsing of the operated lung, inter-lobar fissures, pulmonary vessels, bronchi, and the pulmonary parenchyma to the affected segment were divided and sectioned with endoscopic stapling devices. The resected lung segment was removed in an organ retrieval bag through the utility incision, and followed by dissection of mediastinal lymph node for pathological staging. A 28 F chest tube was then placed through the lowest incision. Rib spreading, rib cutting, and retractor use were avoided in all patients.
Conversion to intubated general anesthesia
The attending surgeon and anesthesiologist decided whether to convert a nonintubated surgery to intubated general anesthesia in cases of ineffective analgesia, persistent hypoxemia (oxygen saturation by pulse oximetry <80%), massive pleural adhesion, unstable hemodynamic status, or intraoperative bleeding requiring a thoracotomy (3,8). For conversion, the surgical wounds were sealed with transparent waterproof dressings (Tegaderm Film, 3M Health Care, Neuss, Germany) after placement of a chest tube to re-expand the collapsed lung. The trachea was then intubated under the guidance of a flexible bronchoscope, followed by insertion of a bronchial blocker for one-lung ventilation without changing the patient’s position.
Postoperative analgesics and care
Postoperative analgesics included continuous epidural infusion of a mixture of bupivacaine (1 mg/mL) and fentanyl (1.25 µg/mL) or intravenous morphine (1 mg/mL) for 1 to 3 days according to the preferences of the patients. Additional analgesics also included oral nonsteroidal analgesics and acetaminophen. Chest radiography was preformed immediately after surgery or on the next morning. The chest tube was removed if no air leak was present and drainage was less than 200 mL in a 24-h period.
Data collection and analyses
Data collection including demographics, operative and anesthetic results, complications and pathologic characteristics were retrieved from the institutional medical records. Intraoperative arterial blood gas analyses were performed 20–30 minutes after the operated lung was adequately collapsed during surgery. The descriptive data are summarized as median (range) and mean ± standard deviation for continuous variables and number (%) for frequency and categorical variables.
Results
From August 2009 to December 2016, nonintubated VATS was performed in 1,069 patients. Among them, 89 patients underwent anatomical segmentectomy for lung cancer. Their data are reported in Table 1. The median patient age was 65 years, and 72 (81%) were women. Half of them (45 patients, 51%) were geriatric older than 65 years. The median body mass index of the patients was 22.0 kg/m2. The median tumor size was 1.2 cm. The pulmonary function of the patients was generally good, with a mean forced vital capacity of 106.8% predicted and forced expiratory volume in one second of 106.6% predicted. However, 28 patients (31%) underwent compromised VATS segmentectomy because of advanced age (26 patients, median: 76.2 years; range, 70–86 years) and/or marginal cardiopulmonary function (3 patients, median forced expiratory volume in 1s predicted, 65%; range, 61.1–65.7%), in spite of their tumors were larger than 2.0 cm (median: 2.5 cm; range, 2.1–3.7 cm).
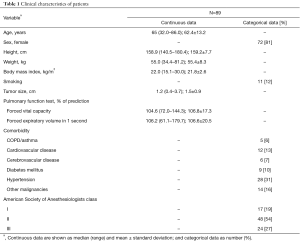
Full table
The anatomical locations of nonintubated VATS segmentectomy are reported in Table 2. Left upper lobe apical trisegmentectomy was the most commonly performed segment, followed by lingular segmentectomy and right upper lobe superior segmentectomy. Most of them were adenocarcinoma (80%) with early pathological stage I (80%), detailed in Table 3.
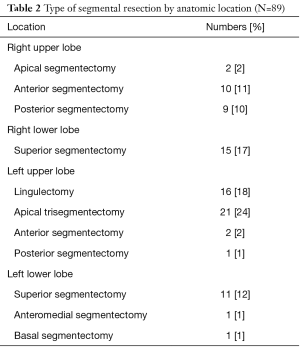
Full table
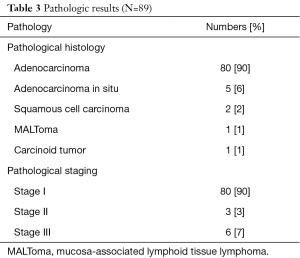
Full table
The operative and anesthetic results are reported in Table 4. The mean duration of anesthesia induction preparation was 14.5 minutes, and the mean operation time was 136.2 minutes. During the operation, the median lowest oxygen saturation by pulse oximetry was 98% (range, 88–100%), and the median highest partial pressure of arterial carbon dioxide during one-lung ventilation was 48.3 mmHg (range, 38–75.4 mmHg). Conversions to tracheal intubation were required in two patients (2%) because of excessive mediastinal and diaphragmatic movement, which increased surgical challenge technically. One was a 69-year-old woman with a body mass index of 30.0 kg/m2 and the other was a 56-year-old man with a body mass index of 26.8 kg/m2. The conversions were accomplished in lateral decubitus position within 20 minutes uneventfully. No patients required conversion to a lobectomy or thoracotomy, neither blood transfusion in any patient.
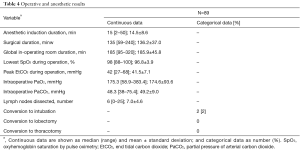
Full table
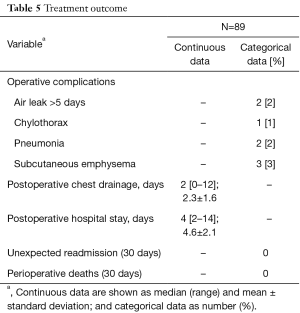
Postoperative results and complications are reported in Table 5. The median postoperative chest tube drainage was 2 days, and the median hospital stay after surgery was 4 days. Prolonged chest tube drainage more than 5 days were noted in three patients because of air leak in two patients and chylothorax in one patient. No deaths occurred in this study.
Full table
Comments
Our results show that nonintubated thoracoscopic segmentectomy is technically feasible and safe for lung cancer patients who are not eligible for a lobectomy or with an intentional purpose for early-stage lung cancer.
Recently, widespread use of computed tomography for lung cancer screening has identified increasing numbers of small lung tumors in patients with high surgical risk or in relatively young adult patients (3,14). To optimizing their quality of life after surgical therapy for NSCLC, a lung parenchyma-sparing segmentectomy is increasingly re-evaluated not only to manage high-risk patients with significantly reduced cardiopulmonary function, but also to treat small early stage NSCLC (tumor less than 2.0 cm), and has been shown a comparable oncological results with the well-established lobectomy (15). In our cohort, compromised VATS segmentectomy was performed in 28 patients (31%) because of advanced age or marginal cardiopulmonary function, even though their tumors were mostly larger than 2.0 cm. The remaining 61 patients received intentional VATS segmentectomy because of small early stage NSCLC (<2.0 cm).
In this cohort, most of the patients completed surgery with adequate sampling of mediastinal lymph nodes, except for the two patients (2%), who required conversion to intubated general anesthesia. The reason to intubation conversion was due primarily to significant mediastinal movements jeopardizing a quiet and safe surgical environment, especially during mediastinal dissection of the bronchovascular structures. In our experience, patients requiring conversion to tracheal intubation were mostly overweight with a body mass index >26 kg/m2. Obesity decreases the functional residual capacity of the lung volume and increases the risk of hypoxemia during anesthesia. Furthermore, a distended diaphragm—especially the hemi-diaphragm below the non-operated lung—usually contracts more efficiently and exaggerates the mediastinal movement in spontaneously breathing patients with an open pneumothorax (16,17). We suggest that obese patients are not ideal candidates for nonintubated thoracoscopic procedures. Consequently, a standby conversion plan should be prepared in advance and performed decisively in cases of large and uncontrollable amplitude of diaphragmatic motion. Tracheal intubation for one-lung isolation in a lateral position may be technically demanding with inherent risks, even by experienced thoracic anesthesiologists. It is our practice to intubate in a lateral position by aid of a flexible bronchoscope, followed by inserting an endobronchial blocker to occlude the operated lung when tracheal intubation conversion indicated. In difficult cases, the patient can also be placed back to a supine position for tracheal intubation in usual manner after inserting a temporary chest tube to re-expand the collapsed lung with wound covering (11).
In our previous study, we showed that nonintubated thoracoscopic anatomical segmentectomy is technically feasible for management of peripheral lung tumors (10). Some concerns may still arise with the use of this technique in NSCLC patients. One major anesthetic concern is how respiratory function is maintained in a spontaneously breathing patient with an open pneumothorax (i.e., in severe hypoxemia and hypercapnia), especially when the surgical duration is usually as long as a VATS lobectomy. Satisfactorily, our patients tolerated the procedure well, and supplemental oxygen by facemask was enough to prevent patients from hypoxemia. Although carbon dioxide rebreathing might occur, these patients had only mild hypercapnia, which was not clinically relevant and resolved soon after termination of sedation. Atelectasis of the dependent lung during mechanical ventilation has been demonstrated to be a major contributing factor in ventilation-perfusion mismatch during one-lung ventilation and predisposes patients to severe hypoxemia (18). Therefore, an alveolar recruitment strategy with application of positive end-expiratory pressure during one-lung ventilation was shown to be substantially effective in decreasing both a right-to-left shunt fraction and dead space ventilation from the dependent lung to improve oxygenation (18,19). We believe that preservation of diaphragm function is pivotal in a single-lung spontaneous breathing scenario, and the lower rate of atelectasis in the dependent lung compared with that in the intubated technique might explain the satisfactory oxygenation results in our patients (10).
The other major concern is whether it is safe and effective to perform a surgical procedure on a nondependent lung—especially when intense pulmonary manipulation and mediastinal lymph node dissections during segmentectomy—might trigger coughing in spontaneously breathing patients. With intrathoracic vagal blockade to abolish the cough reflex, we demonstrated that this procedure was effective during nonintubated anatomic pulmonary resections for lung cancer (10,11). Empirically, retraction of the ipsilateral upper lobe is necessary to expose the intrathoracic vagal nerve. Before lung retraction and injection of local anesthetics, the sedative requirement is transiently increased to prevent triggering a cough reflex (20). Effective vagal blockade abolishes cough reflex and allows us doing extensive pulmonary dissections for more than 3 h. Even though, surgeons should be reminded to gently performing the hilar dissections, because excessive traction of hilar tissue can irritate the contralateral main bronchus, which might induce transient coughing (10).
Conversion from nonintubated VATS to intubated general anesthesia during surgery is a major challenge, which requires the anesthesiologist to perform tracheal intubation in the lateral decubitus position in a timely manner. Indications for a conversion to tracheal intubation include intraoperative bleeding, vigorous mediastinal bleeding, extensive pleural adhesions or persistent hypoxemia or hypercapnia. A well-collaborating teamwork between surgeons and anesthesiologists is of paramount importance to initiate a plan for conversion of tracheal intubation. Once there are any safety concerns, prophylactic conversion to intubated general anesthesia is advised to avoid emergency intubation and complications, especially at the beginning of the learning curve (11). Our low rate of intubation conversion (2%) is similar to others showing continuous improvement of patient safety in nonintubated VATS by judicious patient selection and accumulation of experience in both anesthetic care and surgical skills (21).
Limitation of this study includes its retrospective design and a lack of control group undergoing intubated general anesthesia with positive pressure one-lung ventilation. It is yet early to suggest any specific benefits of the nonintubated technique for VATS segmentectomy, especially in lung cancer patients concerning not only short-term outcomes but also long-term disease-free or overall survival. Even so, our overall experience with low conversion rate from nonintubated to intubated general anesthesia and low complication rate may suffice to suggest that nonintubated VATS segmentectomy can be safely performed in lung cancer patients. Further investigations with good controlled design are helpful to clarify any advantage of the nonintubated technique in lung cancer patients, both in short- and long-term outcomes.
Conclusions
In summary, our experience showed that nonintubated VATS segmentectomy using regional thoracic anesthesia and targeted sedation is technically feasible and safe in lung cancer patients, both in compromised patients and for intentional purposes. While the long-term benefits of nonintubated technique remain unanswered mostly, we suggest that it can be an appealing alternative of intubated general anesthesia in treating lung cancer patients surgically.
Acknowledgments
Funding: This work is supported by a research grant from National Taiwan University Hospital (NTUH104-P08, Dr. Chen), Taipei, Taiwan.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tommaso Claudio Mineo and Marcello Migliore) for the series “Non-intubated Thoracic Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.08.11). The series “Non-intubated Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of National Taiwan University Hospital (201509053RINC). Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
- Ovassapian A. Conduct of anesthesia. In: Shields TW, LoCicero J, Ponn RB. editors. General thoracic surgery. Philadelphia: Lippincott Williams & Wilkins, 2000:327-44.
- Miñambres E, Burón J, Ballesteros MA, et al. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg 2009;35:1056-62. [Crossref] [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic segmentectomy-left upper lobe trisegmentectomy. Ann Cardiothorac Surg 2014;3:208-10. [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Hsu HH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Thorac Cardiovasc Surg 2014;148:e234-5. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Pompeo E. Pathophysiology of surgical pneumothorax in the awake patient. In: Pompeo E. editor. Awake thoracic surgery. Bentham Science Publishers, 2012:9-18.
- Liu YJ, Hung MH, Hsu HH, et al. Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation. Ann Transl Med 2015;3:107. [PubMed]
- Tusman G, Böhm SH, Melkun F, et al. Alveolar recruitment strategy increases arterial oxygenation during one-lung ventilation. Ann Thorac Surg 2002;73:1204-9. [Crossref] [PubMed]
- Tusman G, Böhm SH, Sipmann FS, et al. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 2004;98:1604-9. table of contents. [Crossref] [PubMed]
- Wang ML, Hung MH, Chan KC, et al. Intraoperative multiple intercostal nerve blocks exert anesthetic-sparing effect: A retrospective study on the effect-site concentration of propofol infusion in nonintubated thoracoscopic surgery. Acta Anaesthesiol Taiwan 2016;54:77-80. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
Cite this article as: Hung MH, Wang ML, Cheng YJ, Hsu HH, Chen JS. Nonintubated thoracoscopic anatomical segmentectomy for lung cancer: a single-center experience with consecutive 89 cases. Video-assist Thorac Surg 2017;2:53.


