
Minimally invasive surgery for thymic epithelial tumors: a single institutional experience
Introduction
Minimally invasive surgery (MIS) is increasing to become the preferred surgical approach for anterior mediastinal tumors. Recently, instead of conventional approach (CA) such as median sternotomy and thoracotomy, video-assisted thoracoscopic surgery (VATS) is performed for anterior mediastinal tumor. Some authors reported the advantages of VATS, such as lower blood loss, shorter postoperative pleural drainage duration and length of hospital stay (1-5). However, lack of perspective sensation by two-dimensional view and restricted movements of surgical instruments lead to difficulty of handling the tissues and longer learning curve. Robot-assisted thoracoscopic surgery (RATS) has been adopted to alleviate the shortcoming of VATS recently and some thoracic surgeons reported favorable results of RATS for anterior mediastinal tumors (6,7), and Rückert et al. report that the complete remission rate of MG for RATS is better than that for VATS (8). In this study, the authors reported our experience with MIS including RATS for thymic epithelial tumors and compared the surgical results of MIS with those of CAs.
Methods
Between March 2001 and December 2014, 129 patients underwent surgical treatment for thymic epithelial tumors at Tokyo Women’s Medical University. Of these, 59 patients underwent MIS including VATS and RATS (MIS group) and 70 patients were CA (CA group). The clinical outcomes of matched groups were compared. The study was approved by the Human Research Ethics Board of Tokyo Women’s Medical University (no. 2307 and no. 150801). Informed consent was obtained from all patients.
Surgical procedure of CA and VATS
Endotracheal intubation using a double-lumen endotracheal tube was performed in all cases. There were no patients in CA group underwent operation under general anesthesia with single-lung ventilation. In VATS cases, all procedures were performed via three or four ports under complete thoracoscopic view. Surgical procedures were extended thymectomy, total thymectomy, and hemi-thymectomy. Extended thymectomy was performed in patients with myasthenia gravis (MG). Excepting hemi-thymectomy, both an extended thymectomy and a total thymectomy were performed by bilateral sides approach. Extubation in the operating room was attempted in all patients. Patient with MG was managed in the intensive care unit for post-operative observation overnight.
Surgical procedure of RATS
A robotic system (da Vinci S, Intuitive Surgical, Inc., Sunnyvale, CA, USA) was used. Robotic procedures are usually performed by three surgeons, the surgeon at the console and the two tableside surgeons, who place the trocars and connects them with the robotic arms, changes the robotic instruments, and manipulates additional endoscopic instruments through the auxiliary ports.
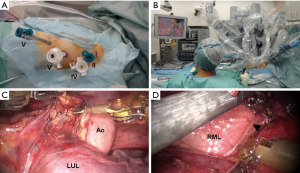
The surgical procedures were performed under general anesthesia with single-lung ventilation using a double-lumen endotracheal tube. Patients were placed in supine position with elevation of the left side (side up at a 30-degree angle) and the ipsilateral arm was placed low. Approach was performed via left side. Total four incisions for camera, instruments, and assist were made (Figure 1A). The camera trocar was first inserted at the fifth intercostal space (ICS), along the anterior axillary line. Pleural adhesion presence was observed by thoracoscopy, which was used only for intrathoracic observation through the first port. The robotic arms were placed in the second (8 mm) and fifth (12 mm) ICS along the anterior-axillary line, and fifth (8 mm) ICS along the midclavicular line. An assist port was placed in the fourth ICS (12 mm) along the mid-axillary line. The patient cart was docked from the right side of the patient. A three-dimensional high definition scope was inserted though the fifth ICS. Both 8 mm ports, in principle, EndoWrist Monopolar Cautery Instrument (Permanent Cautery Spatula, Intuitive Surgical, Inc.) were used as the right arm and EndoWrist Bipolar Cautery Instrument (Fenestrated Bipolar Forceps, Intuitive Surgical, Inc.) was used as the left arm. During the operation, the device of right arm and left arm was replaced. Table surgeons cleaned the lens for securing of the operative field of vision and used ultrasonically activated device for transecting vessels, and were responsible for suction, through the assist port (Figure 1B). Accessory thymic tissue is resected if patient with MG (Figure 1C). Left sided approach allows extensive removal of fat sited in aortopulmonary window and left pericardiophrenic angle (9). Thymic tissue is completely resected along the right phrenic nerve under good visualization (Figure 1D).
The medical records were reviewed for information relating to age, gender, tumor histology, Masaoka stage, Myasthenia Gravis Foundation of America (MGFA) clinical classification, WHO classification, TNM classification, operative details, duration of chest drainage, duration of postoperative hospital stay, and postoperative complications.
Continuous variables were expressed as mean, standard deviation, and categorical variables were expressed as number and percentage. The categorical variables were analyzed by the Chi-square test, and continuous variables were analyzed by the Wilcoxon’s signed rank test. P<0.05 was considered statistically significant. All statistical analyses were performed with JMP software (version 11.2.1, SAS Institute Inc., Cary, NC, USA).
Results
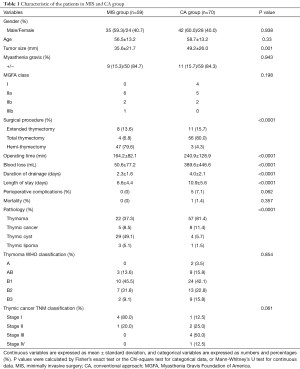
There were 129 consecutive surgical treatments for thymic epithelial tumors at authors’ institution during the study period. The clinical characteristics of the 129 patients are presented in Table 1. Of these, 59 patients (35 males and 24 females) underwent MIS including VATS and RATS, the mean age was 56.5±13.2 years. 70 patients (42 males and 28 females) underwent CA, the mean age was 58.7±13.2 years. The surgical procedures in MIS group included extended thymectomy in 8, total thymectomy in 4, right hemi-thymectomy in 24, and a left hemi-thymectomy in 23 patients. The surgical procedures in CA group included extended thymectomy in 11, total thymectomy in 56, right hemi-thymectomy in 2, and a left hemi-thymectomy in 1 patient.
Full table
The mean tumor size was 35.6 mm in MIS group and 49.2 mm in CA group. Tumor size in MIS group was significantly smaller than those in CA group (P=0.0011). The number of patients with MG was 9 (15.3%) in VATS group and 11 (15.7%) in CA group, with no significant difference.
The operating time, blood loss, duration of drainage, and length of postoperative hospital stay in MIS group were significantly less than those in CA group (P<0.0001). There were no perioperative complications in MIS group. Perioperative complications occurred in 5 patients in CA group: pleural effusion in two patients, right heart injury, postoperative myasthenic crisis, postoperative bleeding, in one patient each. The operative mortality rate was 0% in MIS group, 1.4% in CA group.
The tumor histology in MIS group was thymoma in 22 (WHO classification type AB in 3, type B1 in 10, type B2 in 7, type B3 in 2 patients), thymic cancer in 5 (TNM classification stage I in 4 and II in 1 patient), thymic cyst in 29, and thymic lipoma in 3 patients. In CA group, the tumor histology was thymoma in 57 (WHO classification type A in 2, type AB in 9, type B1 in 24, type B2 in 13, type B3 in 9 patients), thymic cancer in 8 (TNM classification stage I in 1, II in 2, III in 4, IV in 1 patient), thymic cyst in 4, and thymic lipoma in 1 patient. Pathological examination confirmed complete resection of the tumor in all patients.
No recurrence was observed in MIS group but 5 patients in CA group had recurrence. The site of recurrence was pleural cavity in 2 patients occurred in 34 and 6 months after surgery, and the local recurrence at the surgical margin in 3 patients occurred in 10, 32, and 24 months after surgery.
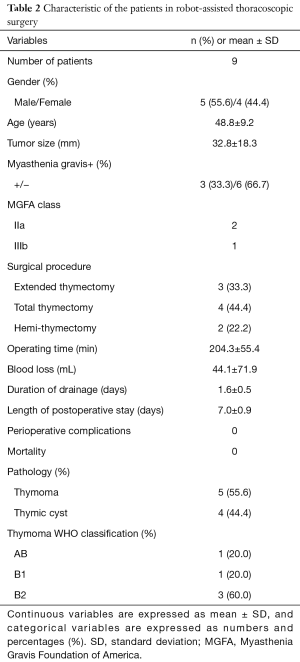
In MIS group, 9 patients (5 males and 4 females) underwent RATS (Table 2). The mean age was 48.8 years. There were extended thymectomy in 3, total thymectomy in 4, and hemi-thymectomy in 2 patients. The mean operative time, blood loss, duration of drainage, and length of postoperative stay were 204.3±55.4 min, 44.1±71.9 mL, 1.6±0.5 days, and 7.0±0.9 days, respectively. The tumor histology was thymoma in 5 (WHO classification type AB in 1, type B1 in 1, type B2 in 3 patients) and thymic cyst in 4 patients. The mean tumor size was 32.8±18.3 mm (range, 16–70 mm) in RATS. Patients with MG were 3 (33.3%). Conversion to VATS or open surgery occurred in no cases. There were no perioperative complications including postoperative myasthenic crisis.
Full table
Discussion
Various tumors, cysts, and other abnormalities relate to the thymus is located in the anterior portion of the mediastinum. Although median sternotomy is accepted as a gold standard for thymic epithelial tumors, due to the technical improvements including new endoscopic instruments in VATS, VATS resection has been gaining in popularity for mediastinal tumor.
In this study, extended thymectomy and total thymectomy were performed mainly in the CA group. On the other hand, the extended thymectomy and total thymectomy by MIS was low with approximately 20%. The recurrence rate of the CA group was high. As a reason, it was that there were a lot of thymoma and thymic cancer and large tumor cases. However, for a resection of small size solid tumor and cystic lesion, MIS is good adaptation. According to previous reports, VATS has become an efficient procedure that results in lower blood loss, shorter drainage duration and postoperative hospital stay compared with those of CA in previous reports (1-5). In this study, the operating time, estimated blood loss, drainage duration, and length of hospital stay in MIS group were all significantly less than those of CA group, these results indicated less invasiveness, similar to previous reports (1-5).
Oncological outcomes are important issues when performing MIS for malignant tumors of thymus. Past reports show that thoracoscopic thymectomy for early stage thymoma presented comparable oncological outcomes to the transsternal approach (2,5,10). The extent of resection (i.e., hemi-thymectomy, total thymectomy, extended thymectomy) is still controversial. There is no evidence to perform total thymectomy for a thymoma without MG. A recent report suggests that the efficacy of partial or subtotal thymectomy for early stage thymoma, such as Masaoka stage I and II thymoma (11). Although further investigation is still required to select the patient, early stage thymoma localized in the left or right side is resected by hemi-thymectomy, resulted in favorable prognosis at the authors’ institute.
Approach side is also under debate, it includes bilateral and unilateral, right or left sided. To date, there is no report comparing these approaches. Schneiter et al. reported that they had changed the surgical access to the left-sided approach by two reasons (12). First, coming through the left thoracic cavity, the right phrenic nerve is protected by the superior vena cava in the delicate region of the right upper thymic horn. Due to a more ventral localization, the left phrenic nerve is easier to identify and preserve. Second, accessory thymic tissue in the aortic-pulmonary window is accessible only from the left side. Mineo et al. reported left sided VATS thymectomy, they prefer this approach because it allows extensive removal of fat sited in aortopulmonary window and left pericardiophrenic angle (9). The authors prefer left-sided approach when performing RATS for these reasons.
The authors have been performing RATS since 2012. Past reports show that the RATS for thymic disease is safe and feasible (6,7,12). RATS has been adopted to alleviate the shortcoming of VATS: it provides a 3-dimensional view, it increases the comfort of the surgeon via the use of EndoWrist instruments (Intuitive Surgical, Inc.), and it enables technically demanding operations with increased freedom for intrathoracic movement. These advantages allow to perform total thymectomy and extended thymectomy by one-sided approach more comfortable and safe. The authors consider that it may lead to less invasive surgery.
As Kumar et al. mentioned, RATS’ limitations should also be borne in mind, which include loss of haptic feedback, limited platform availability, high initial and recurring cost and requirement of special trained team (13). The cost of RATS is a very important issue, Deen et al. reported that RATS costs significantly higher than VATS (14). In Japan, RATS has not been covered by insurance, therefore, RATS is the very expensive surgical approach. Other reasons for the delay in the spread of RATS are proposed by Nakamura et al. (15).
This study has some important limitations. This study was a retrospective single institutional study and included small sample size with various diseases. The results of this study need to be confirmed in a multicenter study with a larger number of patients.
The authors reported in this study reveal that MIS is feasible and safe for both thymic epithelial tumors. MIS including RATS can be standard surgical treatment of anterior mediastinal tumors.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.08.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Board of Tokyo Women’s Medical University (no. 2307 and no. 150801). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Ambrogi V. Left-sided video-assisted thoracic surgery thymectomy. Video-assist Thorac Surg 2017;2:32. [Crossref]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Yano M, Fujii Y, Yoshida J, et al. A Phase II Study of Partial and Subtotal Thymectomy for Thymoma (JART02). World J Surg 2017;41:2033-8. [Crossref] [PubMed]
- Schneiter D, Tomaszek S, Kestenholz P, et al. Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur J Cardiothorac Surg 2013;43:288-92. [Crossref] [PubMed]
- Kumar A, Asaf BB, Cerfolio RJ, et al. Robotic lobectomy: The first Indian report. J Minim Access Surg 2015;11:94-8. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
Cite this article as: Maeda H, Isaka T, Mitsuboshi S, Katagiri S, Sakamoto K, Aoshima H, Kikkawa T, Matsumoto T, Oyama K, Murasugi M, Kanzaki M. Minimally invasive surgery for thymic epithelial tumors: a single institutional experience. Video-assist Thorac Surg 2017;2:47.


