‘Pandora’s box’ of the developing world-perioperative implications of pulmonary infections
Case scenario
In the year 2012, a 10-year-old child with post tubercular lung pathology along with an intra cavitary aspergilloma in the right upper lobe was admitted under our care. He was scheduled for right upper lobectomy by video assisted thoracic surgery (VATS). Standard anaesthetic induction was planned followed by lung isolation with a 28 F double lumen tube (DLT). As we began mask ventilation post anaesthesia induction, the child started to desaturate, with increasing resistance to bag mask ventilation. Lung auscultation revealed bronchospasm and bilateral crepitations. SpO2 decreased to 85% which was initially refractory to bronchodilator therapy, intravenous steroid and deepening the plane of anaesthesia with FiO2 1. Gradually after 5 minutes time, SpO2 showed signs of improvement and reached 100% with FiO2 1. We intubated the trachea with DLT and inflated both the cuffs. SpO2 remained between 90–100%. Bronchoscopy revealed soiling of the left lung field. A provisional diagnosis of spillage of the right upper lobar cavitary contents was made and broncho alveolar lavage was done in an effort to clean the bronchial tree. A decision was made to go ahead with the scheduled surgery as this was the best option for the child. The child had a stormy intraoperative course as one lung ventilation was replaced by intermittent low tidal volume two lung ventilation. The child was shifted to the ICU with the DLT in situ and he was kept on ventilatory support. He had a protracted post-operative course. This was a learning experience for us. Unfortunately, the child in the reference case is just the tip of the iceberg of large number of patients with similar pathologies. We often receive patients with such lesions from peripheral hospitals as there is a dearth of equipment, facility and trained thoracic anaesthesiologist and surgeons at such places. Occasionally, procedures have to be abandoned in these facilities at times even after anaesthesia induction as distorted bronchial anatomy precludes lung isolation. Fortunately, most of the times these patients are well managed in the high volume thoracic surgery set ups. However, complex infective pathologies and their sequelae make these surgeries and their anaesthetic management quite challenging.
Common pulmonary infective conditions presenting for thoracic surgery
A 2012 study published in Lancet observed that one of the leading causes of death in developing countries of the world is respiratory infections including tuberculosis (1). World Health Organization’s 2012 estimate of tuberculosis infection stands at 8.6 million with a fatality of 1.3 million primarily in sub-Saharan Africa (2). India is endemic to tuberculosis with a very high mortality rate of approximately 1,000 individuals per day (3). Multidrug resistant tuberculosis is a growing problem, with about 450,000 new cases in 2012 that contributed to 170,000 deaths (4). Likewise, Aspergillus sensitization and allergic bronchopulmonary aspergillosis also have a very high prevalence in India (5,6). Unfortunately the health sector budgetary allocation in developing countries is not adequate (7).
Tuberculosis
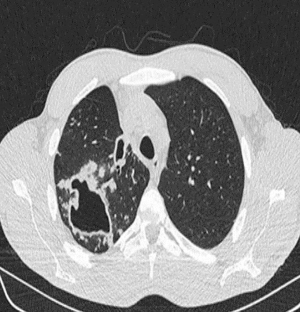
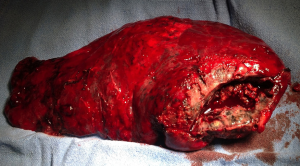
Traditionally thoracic surgery was concentrated on management of tuberculosis (8,9). The magnitude of impact of tuberculosis on mankind was huge and techniques for surgical removal of the diseased lung and obliteration of cavities in the chest were developed to tackle these problems. Role of the surgical team in tuberculosis is mainly for diagnostic purpose, excision of troublesome disease in drug resistant cases, symptomatic relief in haemoptysis, empyema or recurrent chest infections or to deal with one of its long-term sequelae. But, even going by the advances of modern day healthcare, thoracic surgery in case of complicated tuberculosis is quite challenging for the surgeon as well as the anaesthesiologist. Takeda et al. identified aspergillus coinfection, major preoperative comorbidity, long duration of surgery, need of blood transfusion and male gender as risk factors for adverse perioperative outcomes (10). Worldwide, VATS has shown promising results and thoracic surgeons are increasingly confident of the fact that tuberculosis is not a contraindication for VATS. VATS intervention is quite effective in reducing the mortality and morbidity of tubercular sequelae of lung (Figure 1). As a precautionary measure, the preoperative team should take a serious note of any haemoptysis as it can be life threatening at times as the dead space volume of 150–200 mL can be easily filled by blood compromising the airway (11). Establishing the site of the haemoptysis is also important to decide upon lung isolation and bronchoscopy. In areas where conventional lung isolation facilities are unavailable, anesthetist can advance a single lumen tube into the healthy side and continue with volume resuscitation process. The patient can then be shifted to a higher set up for definitive treatment like surgery or bronchial artery embolization.
Empyema thoracis
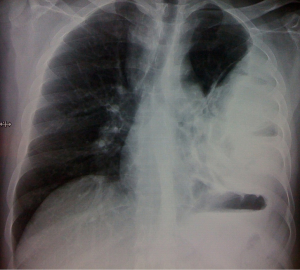
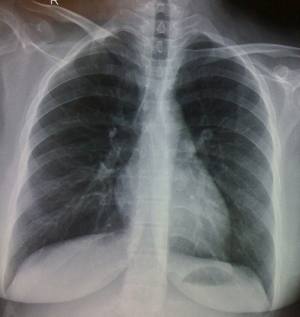
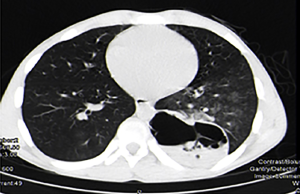
Empyema thoracis (Figures 2,3) can be a complication of pneumonia, tuberculosis or various iatrogenic pulmonary interventions. Despite widespread use of highly effective antibiotics, chronic empyema thoracis (CET) is common worldwide. Corrective procedures like tube thoracostomy, image directed catheters, thoracoscopic drainage, decortication and open drainage have all been employed with success rates varying between 10% to 90% (12). VATS has shown promising results in decortication and adhesiolysis. Perioperative bleeding and air leak are the main concerns in extensive adhesiolysis and decortication. Modern electronic chest drainage systems with suction capability are a boon as it gives the estimate of air leak, drain, helps in lung expansion and early mobility. Talc pleurodesis is employed in an effort to prevent recurrence in patients with repeated effusions.
Aspergillosis
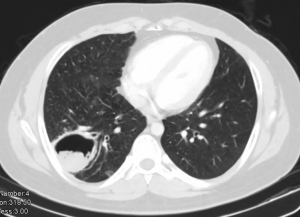
Pulmonary aspergillosis can manifest as invasive aspergillosis, allergic bronchopulmonary aspergillosis (ABPA) or cavitary pulmonary aspergillosis (Figures 4,5). In tuberculosis endemic countries of the world, the healed tubercular cavities act as the much needed nidus for saprophytic colonization of Aspergillus fumigatus. The fungal toxins erode the tissues and can cause haemoptysis when a vessel wall is eroded. Presence of the characteristic fungal ball with ‘air crescent’ in chest X-ray or CT scan clinches the diagnosis. Surgery in the form of pulmonary resection is the mainstay of treatment although intraoperative period can be complicated by dense intra-pleural adhesions, hemorrhage, air leak, disruption of cavity and seeding of the fungus with high morbidity (13-15). Surgical mortality ranges from 0% to 22.6% and reported recurrence rate is 5% (16).
Hydatid cyst
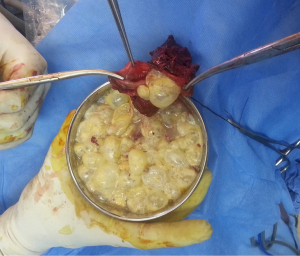
Hydatid disease is a caused by the larvae of Echinococcus. Clinical presentation of cystic echinococcosis (Figures 6,7) varies from asymptomatic disease to acute life threatening emergencies when cyst becomes infected or ruptures. Multiple organ systems may be involved including the brain, lungs, liver, peritoneum, bones, ovaries etc. (17-20). Surgery is the definitive method of treatment. Intraoperative leakage of cyst contents can lead to anaphylaxis and dissemination of infectious scolices causing pleural or bronchogenic hydatidosis. Scolicidal solutions like 1.5% cetrimide–0.15% chlorhexidine (10% Savlon), 3% hydrogen peroxide, 95% ethyl alcohol or 10% polyvinylpyrrolidone-iodine (Betadine) are routinely used along with intravenous steroid. Other non surgical techniques can be image guided percutaneous aspiration, infusion of scolicidal agents etc.
These images demonstrate that the degree of complexity of pulmonary pathology is very challenging in the developing world. Late presentation to the clinician and non compliance to treatment regime are the main causes of such complex case scenarios. Due to late presentation the disease distorts the pulmonary anatomy and physiology. Lung collapse, atelectasis, adhesions, loculated effusions, thick cortex for decortications are frequently encountered. The amount of perioperative blood loss and risk of septicemia are very high.
Our approach at Medanta
Preoperative preparation
Preoperative evaluation and optimization of thoracic surgical patients with sequelae of pulmonary infections should be a team effort amongst the anaesthesiologist, thoracic surgeon, pulmonologist, cardiologist, radiologist and intensivist. Proper planning, coordination and communication are vital for the best outcome.
In developing countries like India, the concept of preventive health checkup is not very common. So, the patient might not have undergone any baseline health checkup for decades. Apart from the routine investigations like complete blood count, coagulation profile, kidney function tests, chest X-ray, ECG etc. thoracic surgery patients need specific systemic evaluation. Liver function might be deranged following anti tubercular therapy. Pulmonary function testing with diffusion studies is a must due to the long standing nature of the pathological process and the possibility of major lung resection to assess the postoperative lung functional capacity. The risk of major blood loss and haemodynamic instability exists in thoracic surgery along with the cardiorespiratory burden of one lung ventilation. A low threshold for cardiac work up is maintained at our Institute considering the demographic profile of the population. Cardiac work up can vary between a simple 2-dimensional echocardiography, exercise stress testing to coronary angiography. Although routinely not done, the use of cardiopulmonary exercise testing (CPET) can be a valuable aid in the perioperative risk stratification in selected group of patients. Ventilation perfusion scan in addition can give an insight into the functional contribution of various lobes and can predict the effect of lobar resection.
Adverse effects of surgery on lung and chest wall mechanics can predispose patients to atelectasis and respiratory infections. Preoperative attention to lung expansion maneuvers like incentive spirometry, deep breathing exercises go a long way in preventing post-operative pulmonary complications. In addition, continuous positive airway pressure (CPAP) therapy should be considered post operatively if necessary.
Pre-existing anemia because of nutritional deficiency, chronic pathology and haemoptysis are common. Extensive dissection also causes major perioperative blood loss, requiring infusions of large amounts of blood products. In our experience, a seemingly benign surgery like decortication can bleed around 800 mL on table and another 500-1000 mL thereafter from the raw surfaces. Adequate quantity of blood products therefore needs to be arranged beforehand.
Preparation for anaesthesia induction
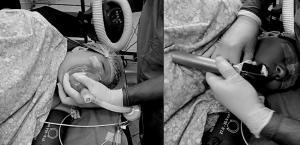
As an additional measure to prevent spillage, we try to keep the affected lobe most dependent till the time the lobar isolation is complete. For this, careful patient and table positioning is required to prevent gravity dependent spillage. In this process, sometimes tracheal intubation in the lateral position might also be required (Figure 8). In our experience intubation in the lateral position is not very difficult in experienced hands. In fact, as the tongue falls down due to gravity, the laryngoscopic view is often better. However, in case of difficulty patient should be made supine immediately and managed further as per difficult airway guidelines.
Preoxygenation is a must in all such patients because of the poor pulmonary reserve. To avoid the risk of atelectasis with 100% oxygen, we generally preoxygenate with 0.8 FiO2 (21). In potential cases of risk of spillage, adequate preoxygenation is followed by anaesthesia inducation with use of succinylcholine or rocuronium as initial relaxant. Post induction, mask ventilation is not done. Once the DLT is secured and cuffs are inflated, only then gentle ventilation is started and tube is checked clinically. Final tube positioning and selective lobar isolation is carried out under fibreoptic guidance with blocker or Fogarty catheter. Once confirmed, nondepolarizing relaxant is continued.
Lung isolation and selective lobar isolation
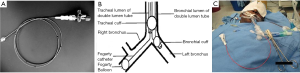
Meticulous planning needs to be done on the mode of securing the airway and its segments. In this regard the anaesthesiologist and the surgeon need to arrive on a consensus, keeping in mind the best interests of the patient. A DLT is the basic lung isolation modality worldwide. In our experience, one area that needs to be addressed in any infective pulmonary pathology is the need for selective lobar isolation on top of standard left from right lung isolation. Sometimes the cavitary lesions of tuberculosis with or without aspergiloma growth communicate with the adjacent bronchial lumen and thereby risking the spillage of its contents to the adjacent lobe and beyond (22). The risk increases further during intraoperative handling. So, along with a DLT which basically isolates the lung, further lobar isolation can be done with the help of bronchial blockers (Coopdech by Smith Medical, Rosmalen, NL, Arndt bronchial blocker by Cook Critical Care, Bloomington, USA) or Fogarty Arterial Embolectomy Catheters by Edwards Lifesciences (23). For example, if the infected cavity is in the left upper lobe, than we can advance the blocker to that lobar bronchus and isolate it from the left lower bronchus. Likewise, a diseased right upper lobe can be isolated by a blocker placed beyond the bronchus intermedius (Figure 9). We routinely resort to selective lobar isolation in suspicious cases. These blockers are removed after thorough suctioning once the surgeon takes the lobar bronchus in the stapler.
Invasive lines and intraoperative monitors
Intraoperative use of invasive monitors like arterial or central venous lines is based on patient’s co morbidities and anticipated volume shifts and loss. From an Indian subcontinent perspective, we tend to rely on a low threshold for invasive lines. Due to complexity of the cases, the duration of surgery and amount of blood loss is generally high. As a protocol we secure an arterial and a central venous line or two wide bore intravenous cannulae for any lung resection surgery beyond segmentectomy. Central venous line is generally not inserted in decortications. Risk of intraoperative arrhythmias is also high especially in the presence of anemia and extensive surgical manipulation due to adhesions. Correction of dyselectrolytemia is a must and antiarrhythmic drugs should be readily available. Advanced dynamic parameters like stroke volume, stroke volume index, cardiac output, extra vascular lung water, central venous oxygen saturation, systemic vascular resistance are helpful and their use should be encouraged in more and more thoracic surgeries (24-26). A word of caution here is that the variations in volumes like stroke volume variation will not be reliable in thoracic surgery or in situations of spontaneously respiration and arrythmias.
Pain management
In absence of contraindications, Paracetamol, nonsteroidal anti-inflammatory drugs and intercostal blocks or simple local infiltration of port sites are used as routine in VATS. Intravenous patient controlled analgesia (PCA) is tailored as per patient’s requirement. In thoracotomies, a more intensive pain control modality like epidural or paravertebral block along with intravenous PCA should be used.
Risk to health care staff and precautions
Various infective conditions of the lung expose the healthcare staff at high risk of cross infection. Although tuberculosis patients coming for surgery are rendered non infectious after couple of weeks to months of antitubercular medicines, an occasional patient with sputum positive tuberculosis will present for segmentectomy or lobectomy. They are labeled as ‘open case of pulmonary tuberculosis’ as they are resistant to antitubercular therapy. Such multi drug resistant tuberculosis patients are a significant health hazard to the health care providers. Anaesthesiologists are particularly vulnerable to cross infection during laryngoscopy and intubation. All cases of tuberculosis must follow the universal barrier protection protocol. The contents of the hydatid cyst or fungal ball of aspergilloma can spill during surgery. This raises a serious biological hazard for the patient and the health care providers. Post operatively operating rooms should be cleaned and disinfected as per local hospital protocol. Open cases of tuberculosis need to be kept in negative pressure rooms and barrier protection needs to be maintained.
Awake video assisted thoracic surgery (AVATS)
Patients with poor respiratory reserve undergo awake VATS under thoracic epidural anaesthesia. We have performed awake VATS including sternotomy for thymoma, segmentectomy, lung biopsy, decortication, evacuation of hemothorax, and retrieval of foreign body. Thoracic epidural anaesthesia was initiated at level T4,5. This was supplemented with intercostal nerve blocks and infusion of dexmedetomidine and fentanyl. With the technique of awake VATS, the benefits of surgery can be extended to the select group of patients with severely compromised cardiopulmonary status who would otherwise be unfit for general anaesthesia (27,28). However patient selection needs to be meticulous for AVATS. Preoperative and intraoperative communication and planning between the anaesthesiologist and the surgeon is a must. AVATS allows early expectoration as the respiratory capacity of the patient is maintained. This allows adequate lavage in case of a cavitary lesion communication with a bronchus. AVATS should be offered in high volume, tertiary centres.
The way forward
If as a country, we thrive to reduce the incidence of serious pulmonary diseases, primordial prevention should be the answer. Better understanding of the epidemiology, pathogenesis, improved public health and sanitation has tremendous role towards that goal. Budgetary allocation must go up to meet the need of healthcare expenditure. More and more numbers of specialized centres dealing with such complex surgeries in smaller cities is the need of the hour. Cost factor needs to be considered as a major chunk of the population of countries like India are poor and unfortunately such diseases have a special inclination to the socioeconomically downtrodden. As perioperative health care provider, the anaesthesiologist and the surgical team should understand the safety goals and know the various techniques of lung isolation and if necessary selective lobar isolation. We are happy that after the index case described earlier, we had our protocol in place and the event is still the only one clinically relevant perioperative spillage we have encountered. To conclude, we would like to recommend to the reader a few pearls of management:
- Infectivity of the patient should be considered in the preoperative work up;
- The anaesthesiologist should review with the surgical team whether the patient needs general anaesthesia or surgery can be done with regional blocks;
- The entire team should arrive at a consensus on need of broncho alveolar lavage, rigid/flexible scopy etc.;
- Preoxygenation is a must;
- Mask ventilation before intubation may be done away with in select group of patients with risk of spillage;
- Intubation in lateral position can be considered along with positioning of the patient in such a way that the affected lobe is in the most dependent position;
- Selective lobar isolation should be considered in all patients with risk of spillage;
- Video assisted technique is very effective in the surgical management of infective lung pathologies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci and Ilaria Righi) for the series “Minimally Invasive Management of Infectious Pleuropulmonary Diseases” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.07.04). The series “Minimally Invasive Management of Infectious Pleuropulmonary Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [Crossref] [PubMed]
- World Health Organization. Global tuberculosis report 2013. Geneva: World Health Organization; 2013.
- TB India 2013: RNTCP annual status report. Available online: http://tbcindia.nic.in/pdfs/TB India 2013.pdf. Accessed 24 December 2013.
- Bryce J, Black RE, Walker N, et al. Can the world afford to save the lives of 6 million children each year? Lancet 2005;365:2193-200. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D, et al. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: systematic review and meta-analysis. Int J Tuberc Lung Dis 2009;13:936-44. [PubMed]
- Agarwal R, Khan A, Gupta D, et al. An alternate method of classifying allergic bronchopulmonary aspergillosis based on high-attenuation mucus. PLoS One 2010;5:e15346 [Crossref] [PubMed]
- Chaulet P. After health sector reform, whither lung health. Int J Tuberc Lung Dis 1998;2:349-59. [PubMed]
- Pezzella AT, Fang W. Surgical aspects of thoracic tuberculosis: a contemporary review--part 1. Curr Probl Surg 2008;45:675-758. [Crossref] [PubMed]
- Pezzella AT, Fang W. Surgical aspects of thoracic tuberculosis: a contemporary review--part 2. Curr Probl Surg 2008;45:771-829. [Crossref] [PubMed]
- Takeda S, Maeda H, Hayakawa M, et al. Current surgical intervention for pulmonary tuberculosis. Ann Thorac Surg 2005;79:959-63. [Crossref] [PubMed]
- Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care.Illustrative case 7: assessment and management of massive haemoptysis. Thorax 2003;58:814-9. [Crossref] [PubMed]
- Lee KS, Im JG, Kim YH, et al. Treatment of thoracic multiloculated empyemas with intracavitary urokinase: a prospective study. Radiology 1991;179:771-5. [Crossref] [PubMed]
- Rergkliang C, Chetpaophan A, Chittithavorn V, et al. Surgical management of pulmonary cavity associated with fungus ball. Asian Cardiovasc Thorac Ann 2004;12:246-9. [Crossref] [PubMed]
- Babatasi G, Massetti M, Chapelier A, et al. Surgical treatment of pulmonary aspergilloma: Current outcome. J Thorac Cardiovasc Surg 2000;119:906-12. [Crossref] [PubMed]
- Demir A, Gunluoglu MZ, Turna A, et al. Analysis of surgical treatment for pulmonary aspergilloma. Asian Cardiovasc Thorac Ann 2006;14:407-11. [Crossref] [PubMed]
- Kim YT, Kang MC, Sung SW, et al. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294-8. [Crossref] [PubMed]
- Yalçinkaya I, Er M, Ozbay B, et al. Surgical treatment of hydatid cyst of lung: review of 30 cases. Eur Respir J 1999;13:441-4. [Crossref] [PubMed]
- Khurana S, Das A, Malla N. Increasing trends in seroprevalence of human hydatidosis in North India: A hospital-based study. Trop Doct 2007;37:100-2. [Crossref] [PubMed]
- Tiwary AK, Tiwary RN. Hydatid disease in Chotanagpur region of South Bihar. Indian J Surg 1988;50:14-8.
- Nepalia S, Joshi A, Shende A, et al. Management of echinococcosis. J Assoc Physicians India 2006;54:458-62. [PubMed]
- Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: Physiologic Basis, Benefits, and Potential Risks. Anesth Analg 2017;124:507-17. [Crossref] [PubMed]
- Pfitzner J, Peacock MJ, Tsirgiotis E, et al. Lobectomy for cavitating lung abscess with haemoptysis: Strategy for protecting the contralateral lung and also the non-involved lobe of the ipsilateral lung. Br J Anaesth 2000;85:791-4. [Crossref] [PubMed]
- Sharma A, Sinha S, Khanna S, et al. A novel technique to prevent endobronchial spillage during video assisted thoracoscopic lobectomy. Ann Card Anaesth 2014;17:164-6. [Crossref] [PubMed]
- Zhang J, Chen CQ, Lei XZ, et al. Goal-directed fluid optimization based on stroke volume variation and cardiac index during onelung ventilation in patient s undergoing thoracoscopy lobectomy operations: a pilot study. Clinics 2013;68:1065-70. [Crossref] [PubMed]
- Lema-Tome M, Alvarez M, Hernandez G, et al. Modifications of stroke volume variation and stroke volume index during thoracic surgery with onelung ventilation: 4AP49. Eur J Anaesthesiol 2014;31:64. [Crossref]
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol 2013;26:31-9. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery-extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]
- Jancovici R, Lang-Lazdunski L, Pons F, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg 1996;61:533-7. [Crossref] [PubMed]
Cite this article as: Khanna S, Das J, Mehta Y, Khan AZ. ‘Pandora’s box’ of the developing world-perioperative implications of pulmonary infections. Video-assist Thorac Surg 2017;2:44.