
Hybrid approach for VATS pulmonary resection
Introduction
In many institutions, many different surgical VATS procedures are performed, and this has resulted in there being no clear definition or standard for this surgical procedure. Swanson et al. (1) defined VATS lobectomy “to encompass a true anatomic lobectomy with individual ligation of lobar vessels and bronchus as well as hilar lymph node dissection or sampling using the video screen for guidance, two or three ports, and no retractor use or rib spreading.” We call this procedure thoracoscopic VATS. Conversely, Okada et al. (2) proposed the procedure of hybrid VATS, in which muscle-sparing minithoracotomy (incision, 4–10 cm) is combined with video assistance primarily using direct visualization for lung resection. We call this procedure hybrid VATS. In this article, we present the features of each procedure and introduce the usefulness of hybrid VATS procedures, especially in segmentectomy.
Thoracoscopic VATS or hybrid VATS
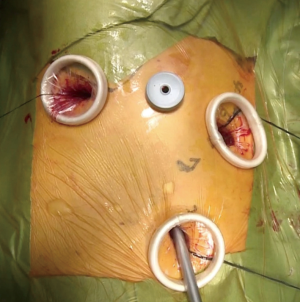
In our thoracoscopic VATS, four ports (three 2-cm ports at the 4th and 7th intercostal spaces at the anterior axillary line and at the incision in the 5th intercostal space at the posterior axillary line, with a 5-mm port in the 3rd or 4th intercostal space at the mid-axillary line) are used with thoracoscopic vision alone (Figures 1,2). In our hybrid VATS (Figure 3), two 2-cm ports (4th and 7th intercostal spaces at the anterior axillary line) are combined with muscle-sparing mini-thoracotomy with a slightly opened metal retractor (incision length of 4–8 cm in the 4th intercostal space at the anterior for the upper or middle lobe resection (Figures 3A,4), or in the 5th intercostal space at the posterior axillary line for the lower lobe (Figures 3B,5) including segmentectomy with direct vision and thoracoscopic vision. Both procedures are performed by an operator, one assistant, and a scopist. The operator usually stands at the ventral side of the patient in thoracoscopic VATS. In hybrid VATS, the operator usually stands at the ventral side of the patient when approaching the upper or middle lobe, or at the dorsal side of the patient when approaching the lower lobe. Forceps (GEISTER Medizintechnik GmbH, Tuttlingen, Germany), electrocautery, and ultrasonic scissors (HARMONIC ACE, ETHICON ENDO-SURGERY, LLC., Cincinnati, OH, USA) were used.




VATS lobectomy has been compared with conventional open lobectomy in several studies (6,7). Since data from prospective randomized studies of VATS lobectomy and open lobectomy are lacking, several propensity score-matched studies were performed (8-11). These studies suggested that the VATS approach showed a lower incidence of complications and no inferiority in overall survival, disease-free survival, and local relapse. In our unpublished propensity score-matching analysis comparing VATS and open lobectomy, shorter operative time and duration of drainage, along with less blood loss and fewer postoperative complications, were seen with VATS, while there was no significant difference in 5-year survival between VATS and open lobectomy in cases of clinical stage I lung cancer.
With respect to hybrid VATS (direct and video vision) and thoracoscopic VATS (video vision alone), hybrid VATS has been reported to have a shorter operative time and no differences in amount of bleeding, chest tube duration, and all complications in a propensity-matched analysis (12), along with no significant differences in overall and disease-specific survivals. In their comparison of VATS with muscle-sparing thoracotomy (MST), which is similar to the hybrid VATS procedure, Kuritzky et al. (13) showed that the only differences between the groups were in operative time (favoring MST) and hospital days (favoring VATS), with no differences in major outcomes, such as postoperative complications, disease-free survival, and overall survival. In their review, Jheon et al. suggested that, in surgery for lung cancer, the most important issue is not whether the VATS technique is thoracoscopic or hybrid, but that the procedure be safe while complying with oncological standards (14).
VATS segmentectomy
Recently, to minimize lung resection volume without decreasing curability, curative segmentectomy has been attractive and increasingly performed to treat small non-small-cell primary lung cancers (15-18). VATS segmentectomy is an ideal surgical procedure to treat primary lung cancer from the perspective of minimally invasive surgery. Our segmentectomy procedure was previously described (19).
The most important issue in segmentectomy is to maintain a sufficient margin from the tumor. In a study by Khullar et al. (20), sublobar resection was shown to be inferior to lobectomy because of inadequate lymphadenectomy and positive sublobar resection margins. To maintain an adequate margin, Tsubota et al. (21) suggested “extended segmentectomy”, which involves placing the resection line on the segment beyond the affected one. A multicenter, prospective study confirmed that there were no positive margins when the margin distance was greater than the maximum tumor diameter (22).
Iwata et al. (19) classified segmentectomy into two types: a simple type, involving resection of only one intersegmental plane, such as superior or lingual segmentectomy; and a complicated type, involving resection of at least two intersegmental planes, such as anterior segmentectomy. The technical considerations and outcomes of thoracoscopic VATS segmentectomy for mainly the simple type have been reported by D’Amico (23). In addition, Ohtaki et al. (24) demonstrated that thoracoscopic VATS segmentectomy was less invasive than open segmentectomy. Recently, Ghaly et al. (25) showed that thoracoscopic VATS segmentectomy for clinical stage I NSCLC decreased the length of stay and pulmonary complications. Furthermore, 5-year disease-free survival and overall survival were better with VATS than with open thoracotomy. However, the median surgical margin was 1.4 (0.6–2) cm and 1.5 (0.8–2.7) cm in VATS and in thoracotomy, respectively, while the locoregional recurrence rate was 7.7% and 12.7%, respectively. In several Japanese studies, locoregional recurrence rates were 0–5% (26-28). We think that it is important to determine whether thoracoscopic VATS segmentectomy without direct visualization or rib spreading can achieve an adequate surgical tumor-free margin, especially for complicated types (22). Delicate surgery is required to keep the surgical margin in a complicated type segmentectomy. Therefore, the hybrid approach seems to be reasonable for obtaining sufficient surgical margins.
Acknowledgments
The authors would like to thanks Ms Yoshimi Hayashi and Tomoko Kamiya for their secretarial support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “Video-assisted major thoracic procedures: Approaches” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.06.01). The series “Video-assisted major thoracic procedures: Approaches” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-Assisted Thoracic Surgery Lobectomy: Report of CALGB 39802—A Prospective, Multi-Institution Feasibility Study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [Crossref] [PubMed]
- Yamamoto H, Shirahashi K, Matsumoto M, et al. Our thoracoscopic VATS right lower lobectomy for primary lung cancer. Asvide 2017;4:269. Available online: http://www.asvide.com/articles/1578
- Yamamoto H, Shirahashi K, Matsumoto M, et al. Our hybrid VATS right upper anterior segmentectomy for metastatic lung cancer. Asvide 2017;4:270. Available online: http://www.asvide.com/articles/1579
- Yamamoto H, Shirahashi K, Matsumoto M, et al. Our hybrid VATS left anterior (S8) and lateral (S9) basal segmentectomy for primary lung cancer. Asvide 2017;4:271. Available online: http://www.asvide.com/articles/1580
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Iwata H. Minimally invasive pulmonary surgery for lung cancer, up to date. Gen Thorac Cardiovasc Surg 2013;61:449-54. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Iwata H, Shirahashi K, Yamamoto H, et al. Propensity score-matching analysis of hybrid video-assisted thoracoscopic surgery and thoracoscopic lobectomy for clinical stage I lung cancer. Eur J Cardiothorac Surg 2016;49:1063-7. [Crossref] [PubMed]
- Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by Video-Assisted Thoracic Surgery vs Muscle-Sparing Thoracotomy for Stage I Lung Cancer: A Critical Evaluation of Short- and Long-Term Outcomes. J Am Coll Surg 2015;220:1044-53. [Crossref] [PubMed]
- Jheon S, Yang HC, Cho S. Video-assisted thoracic surgery for lung cancer. Gen Thorac Cardiovasc Surg 2012;60:255-60. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for lung cancer. Gen Thorac Cardiovasc Surg 2008;56:151-7. [Crossref] [PubMed]
- Iwata H, Shirahashi K, Mizuno Y, et al. Surgical technique of lung segmental resection with two intersegmental planes. Interact Cardiovasc Thorac Surg. 2013;16:423-5. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection vs. Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Thoracic Surgery Study Group of Osaka University. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- D’Amico TA. Thoracoscopic segmentectomy: technical considerations and outcomes. Ann Thorac Surg 2008;85:S716-8. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586-93. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Prognostic predictors in non-small cell lung cancer patients undergoing intentional segmentectomy. Ann Thorac Surg 2012;93:1788-94. [Crossref] [PubMed]
Cite this article as: Yamamoto H, Shirahashi K, Matsumoto M, Miyamaoto Y, Doi K, Iwata H. Hybrid approach for VATS pulmonary resection. Video-assist Thorac Surg 2017;2:40.


