Full thoracoscopic fissure based technique for major pulmonary resections: rational and basic considerations
Our team, as well as some others, has been involved in thoracoscopic major pulmonary resections (MPR) for more than 20 years (1), at a time where these techniques were raising some skepticism (2). In the beginning of this adventure, the instruments were rudimentary and the image quality was poor. We have used various video-assisted methods and none of them proved satisfactory. We found, as written by other authors (3), that the so-called access incision was giving a false sense of security. Meanwhile our colleagues from other specialties were performing more and more complex procedures using pure laparoscopic techniques without assistance of a utility incision. By observing what was going on, we came to the following statements:
- Major procedures can be performed efficiently and safely using a full endoscopic approach;
- The classical way of performing a lobectomy through a thoracotomy, with adequate exposure and full dissection of the fissure, is logical, efficient and safe and has advantages, particularly in terms of understanding of the anatomy;
- Therefore, it must be possible to perform a MPR partly basing on a conventional opening and dissection of the fissure, but using a full thoracoscopic approach.
Thus, from 2007 to now, we have performed 1,050 full thoracoscopic lobectomies and segmentectomies using this this technique whose rational and basics will be reported in this article. Precise description of most thoracoscopic MPR can be found elsewhere (4) and our results have been published (5-10).
Rational
The full endoscopic technique—described here—is an adaptation of the so-called “posterior approach” published by Walker et al. as early as 1993 (11,12) but getting rid of the utility incision. The posterior approach can be summarized as a conventional technique where the surgeon stands in the patient's back and has a familiar vision, comparable to open thoracotomy. William Walker now suggests to rather use the term “fissure-based technique”, which is preferable. Indeed, the main principle of this approach is a wide opening of the fissure and an extensive dissection of the arterial branches, so that the risk of anatomical misjudgment is minimized.
Whenever possible, the diameter of trocars is reduced to the minimal, i.e., 5 and 3 mm. The reason of working with small instruments is twofold: (I) minimizing intercostal trauma and (II) enhancing the precision of dissection because instruments tips are better suited to the dimensions of the anatomical elements that are dissected.
This technique raises three questions: (I) Why using multiple ports? (II) Why not using a utility incision? (III) Why using a fissure first technique?
Question 1: “Why using multiple ports at a time where more and more publications deal with single-port technique?” Our response is multiple:
- “To be well exposed is a prerequisite for the success of surgical procedure”. This is what surgeons have learned and there is no reason why this principle should not be valid also in thoracoscopic surgery. As exposure cannot be obtained by hands or by large conventional retractors, it should be achieved by other means, i.e., multiple instruments. Surgeons can collaborate with instruments manufacturers to design small diameter devices or even “no-trocar” instruments that have minimal impact on the chest wall.
- Surgery cannot be blind, especially when dealing with major vascular hazards. Having an optimal vision of the target requires keeping a perfect image. This means the endoscope cannot be soiled, which inevitably occurs when the field is not enough cleared off the lung. This again requires adequate exposure and appropriate instruments.
- Finally, as robotic surgeons promote the robot because—out of several advantages—it offers 4 ports, it is unclear what prevents thoracoscopic surgeons operating with the same principles. In the past years, many technical articles have reported approaches using single port, 2 ports or 3 ports, as if the number of trocars was a major concern. This is most likely a false debate (13,14). The only concern is being well exposed, whatever the number of ports. However, although not demonstrated, postoperative pain can be related to the number of ports but also to their diameter. For this reason, we do not limit the number of instruments but, whenever possible, use small diameter ones to minimize the intercostal trauma. Many tasks can indeed be done with micro-instruments. In addition, it could be preferable to have several small diameter ports rather than 2 to 3 large ports that may exert excessive torque with its inherent intercostal nerve compression.
Question 2: “Why not using a utility incision, since an extraction incision will be needed anyway” is also a frequently asked question. Our response is again multiple:
- When using only dedicated endoscopic instruments, an access incision is useless.
- We previously used a video-assisted approach and a utility incision. We have found it does not contribute to safety. Indeed, the site of the incision is usually chosen for a dedicated step such as hilar dissection or fissure division, so that there are always some steps of the procedure for which the incision location is not suitable. In the numerous published articles, photographs or line drawings showing many different locations for the utility incision illustrate this issue. In addition, in case of intraoperative complication, enlarging the incision may be problematic since it is often not on the line of a posterolateral thoracotomy, which is the most appropriate incision in case of emergency.
- Finally, in other surgical specialties, complex procedures with major vascular dissection are carried out laparoscopically without help of a utility incision. These procedures are yet accepted.
Question 3: “Why preferring a fissure first technique?”
The popular so-called anterior approach with fissure-last technique offers several advantages. When the fissure is partly of totally fused (Craig-Walker grade III or IV) (15), it avoids a tedious step, i.e., its opening and its potential side effects such as oozing, hemorrhage and postoperative air leak. This results in a procedure that is usually fast and safe because stapling minimizes blood loss and air leaks. It is however unclear if this technique is done by conviction (and if, so, this means that some ancient surgical principles, like extensive vascular dissection and bronchial clearance, are eventually not valid) or by necessity because the quality of exposure or instrumentation or both does not permit performing these steps safely. The recent re-discovery of the interest of a fissure first technique (16,17) may be partly related to the fact that with growing experience and technical progresses, this approach is actually considered easier than in the past.
Basic considerations
Ergonomics
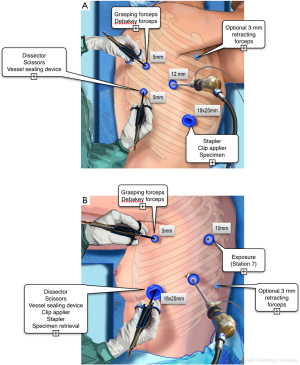
Ergonomic problems are neglected by thoracic surgeons (18). Knowing ergonomic risks has however a major impact on physical comfort of the surgeon and on patient’s safety. The classical “triangulation” position of ports, with arms abducted, should be avoided because it provokes major shoulder discomfort. Whenever possible, it is more comfortable manipulating the working instruments from the back or from the front, depending on the resection to be performed (Figure 1). The opposite side is used only when necessary, for insertion of a lung retractor or a suction device or a stapler.
A mechanical or motorized scope holder, according to the surgeon preference, holds the scope. Its position should be shrewdly chosen so that it does not conflict with instruments. Endoscopic instruments and trocars are placed on a dedicated rack and the conventional thoracic instruments are prepared on a separate table.
Three monitors are used for the surgeon, its assistant and the scrub nurse, their position being adapted throughout the procedure for optimal vision.
Ports
The location of ports depends on the patient’s morphological type and on the surgeon’s habits and preferences. For instance, we prefer performing left resections with the instruments coming from the front (Figure 1), while right-sided resections are performed with a combination of dissection from the back and from the front. Other surgeons may feel more comfortable with another approach and different positioning. The only recommendations we would give are the following:
- Inserting the scope in the mid-axillary line, in the 6th or 7th intercostal space, depending on the patient’s morphological type, because of the need to have an overall view of the pleural cavity.
- Whenever possible, avoiding large trocars in the posterior axillary line as intercostal spaces are there tighter.
- Haemostasis of the trocar path should be as meticulous as possible. An insidious bleeding frequently comes from the trocar hole.
- If a large size trocar is used, mostly anteriorly, it must be tightly sutured to prevent postoperative pulmonary hernia. Passing a chest tube through this incision should be avoided as this can weaken muscular healing.
Operating with the optimal angle of vision
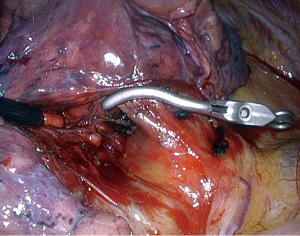
What is the viewing angle of the endoscope used for thoracoscopic MPR is a frequently asked question. Some surgeons prefer a straight viewing scope (0°) because it gives a more natural vision. Others favor oblique viewing scopes, usually 30°, to avoid tangential vision when the target is remote from the endoscope insertion port. It is however rare that one of these two choices remains ideal all along the procedure, especially when moving from close-up to overall views. This is the reason why having the possibility to switch from a straight viewing angle to an oblique one with a single endoscope should be the best option (19). We use the Olympus LTF. This endoscope is a 10-mm rigid one but has a flexible distal part housing the chip at its tip. The distal part can be deflected from 0° to 100° up-down and right-left or any combination of these movements thanks to two levers located on the handle (Figure 2). Once the appropriate angle has been chosen, it can be locked. It is possible to switch from a direct view to a bird-eye vision in just one action. We are using this scope during all our thoracoscopic MPR for more than 10 years. It is especially helpful during lymph node dissection (Figure 3).


Keeping a stable image
From the early beginning of our experience in thoracoscopic surgery, we have worked with a scope holder for three reasons: (I) it allows avoiding a shaking picture; (II) the operative field remains hand-free, preventing instruments conflicts and hands crowding over the patient’s chest; (III) the assisting surgeon can concentrate on other tasks than holding the endoscope. However, few thoracic surgeons are familiar with this possibility. The holder can be mechanical (Figure 4) or robotized (Figure 5). It can be fixed onto the operating table rail. Its long and thin arm saves space around the patient’s chest and avoids clashes with instruments. The system can move the endoscope forward and backward, up and down and laterally. The combination of the movements of this scope holder and of the view angles of the LTF thoracoscope makes it possible to reach most targets without manipulating the scope.

Operating with a clean lens
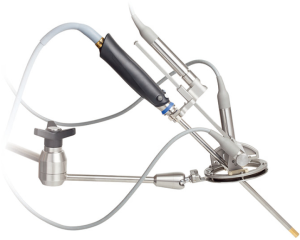
One of the more frequently encountered problems during thoracoscopic procedure is the soiling of the endoscope tip by blood dripping along the trocar sheath. We use a specific trocar, i.e., a skirt mounted at the tip, which deflects the blood drops (Figure 6). This simple tool is very efficient and is now used during all our thoracoscopic procedures.
Lens fogging is also a well-known concern and is a real impediment to a clear vision. It is caused by condensation due to temperature difference between the operating room and the patient’s thoracic cavity. Until now, the most efficient system we have used is the built-in warming system in Olympus endoscopes. A fog-free element is located at the back of the leading-end objective lens. It is combined with a sensor that constantly monitors the temperature. The fog-free element warms the lens and maintains it at the predetermined temperature, i.e., 39 °C. Since we are using this system, we don’t need any anti-fogging solution or any other heating system.
Instruments
Advanced thoracoscopic procedures can be performed with conventional thoracotomy instruments, laparoscopic instruments or dedicated ones.
Conventional instruments can only be inserted via an access incision or through a large port. Although they may look familiar to the thoracic surgeons, their design does not match the requirements of a sharp endoscopic dissection. Their tip is usually too big compared to the size of dissected structures.
Laparoscopic instruments fit the 5 and 10 mm trocars and are better suited to endoscopic surgery. They have also some drawbacks: their design does not allow applying much force on the jaws. One of the consequences is that some delicate tasks like grasping a vascular sheath is almost impossible. Their shaft is too long and their pistol handle is not ergonomic. After years of uncomfortable work with these instruments, we have decided to develop a dedicated range of instruments whose features may be summarized as such: (I) short shaft; (II) in line pen-style handle; (III) precise and strong jaws. This results in a more ergonomic, more natural and more precise dissection (Figures 7,8).

Exposure and lung retraction
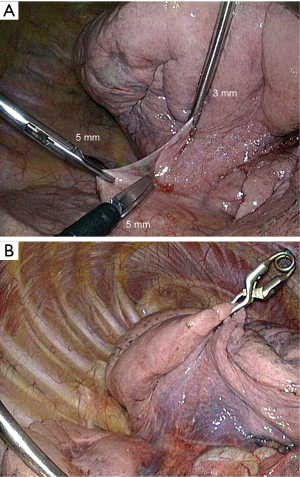
The most efficient and most natural way to retract the lung is by mean of a 5- or 10-mm forceps. However, though it is sometimes necessary, the use of a forceps has two drawbacks: (I) it requires an additional port; and (II) it frequently tears the parenchyma, causing oozing or even hemorrhage and air leak. We use either a 3-mm grasping forceps (Figure 9A) or a miniaturized lung forceps that can be released inside the chest cavity (Figures 9B,10), whose features and interest have been reported (23).

Fissures
The access to the branches of the pulmonary artery in the fissure is straightforward or difficult depending on whether the fissure is separated (grades I and II) (Figure 11) or fused (grades III and IV) (Figures 12,13) (15). Opening a largely fused fissure may be a tedious step of the procedure. The main concern is that opening and dissecting the fissure can cause some minor oozing that is troublesome during a thoracoscopic operation where the operative field must remain as dry as possible to keep optimal vision. One of the keys of a bloodless dissection is to progress step by step, from the periphery to the hilum. We have found thin instruments such as ultrasonic (25) or electrothermal bipolar shears (26,27) to be less cumbersome and as effective as stapler for the division of the external part of the fissure (Figure 13). For the inner thick part of the fissure, stapling is, however, required .
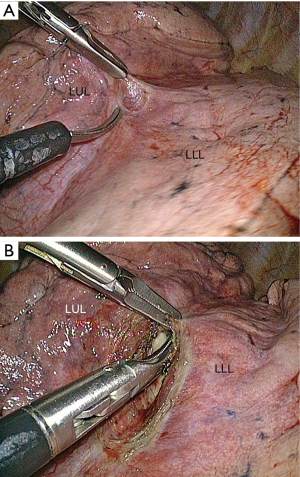

With a direct viewing telescope, the division of the fissure can be difficult because its length may make sharp vision on both of its extremities almost impossible. A deflectable tip thoracoscope is of great help during this step because it allows a bird’s-eye view of the whole fissure throughout the dissection. When the fissure is thick and totally fused, opening it becomes almost impossible and may lead to major air leaks. The “tunnel technique” reported by Decaluwé et al. is an interesting alternate solution in these rare cases (17).
Vascular control
Avoiding bleeding and massive vascular injury is a constant concern throughout the procedure. However, in patients presenting with a regular anatomy, the risk of major vascular injury is low because of the close-up dissection and the camera magnification. Dissection becomes riskier when neoplastic or inflammatory lymph nodes are present and prevent opening the vascular sheath which is adherent to the underlying vessel. These situations may lead to abandon and convert to an open procedure.
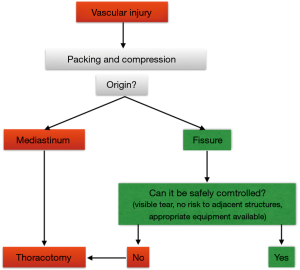
Massive hemorrhage
A massive bleeding that is not controllable by thoracoscopy should lead to introduce gauzes through the larger port and temporary control bleeding by packing, while the surgeon convert to thoracotomy (Figures 14,15). This outlines the importance of having the conventional thoracic instruments ready on a separate operative table.

Minor hemorrhage
A 5-mm vascular clamp must be available. We prefer using throw-off vascular bulldog clips (Figures 16,17). They are used for controlling a hemorrhage or during the temporary occlusion of a vessel. They are inserted through a 12-mm trocar using a dedicated applier that is withdrawn after the device has been applied. The same instrument is used for retrieving the device.

Conclusions
In summary, the full thoracoscopic approach to MPR is one technique among many other ones. Nothing proves it is superior or inferior to these and several publications reporting the results of other techniques are reliable. However, the use of multiple ports greatly facilitates exposure and, consequently, enhances vision. The lack of access incision counterbalances the potential chest wall trauma related to the 3 or 4 trocars, which, besides, are of small diameter. In the future, technology will help reducing the size and ergonomics of hand-instruments. Ongoing developments of motorized and robotized tools will permit overcoming some of the limitations of current straight instruments.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “Video-assisted major thoracic procedures: Approaches” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.06.02). The series “Video-assisted major thoracic procedures: Approaches” was commissioned by the editorial office without any funding or sponsorship. DG is consultant for Delacroix-Chevalier instruments manufacturer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yan TD, Cao C, D'Amico T, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Yim AP. Video-Assisted Thoracic Lung Surgery: Is There a Barrier to Widespread Adoption? Ann Thorac Surg 2010;89:S2112-3. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [Crossref] [PubMed]
- Gossot D. Atlas of endoscopic major pulmonary resections. Paris: Springer-Verlag; 2010.
- Fournel L, Zaimi R, Grigoroiu M, et al. Totally thoracoscopic major pulmonary resections: an analysis of perioperative complications. Ann Thorac Surg 2014;97:419-24. [Crossref] [PubMed]
- Gossot D. Technical tricks to facilitate totally endoscopic major pulmonary resections. Ann Thorac Surg 2008;86:323-6. [Crossref] [PubMed]
- Gossot D, Ramos R, Brian E, et al. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg 2011;12:529-32. [Crossref] [PubMed]
- Gossot D, Zaimi R, Fournel L, et al. Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations. J Thorac Dis 2013;5:S200-6. [PubMed]
- Ramos R, Girard P, Masuet C, et al. Mediastinal lymph node dissection in early-stage non-small cell lung carcinoma: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg 2012;41:1342-8; discussion 1348. [Crossref] [PubMed]
- Ramos R, Masuet C, Gossot D. Lobectomy for early-stage lung carcinoma: a cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surg Endosc 2012;26:431-7. [Crossref] [PubMed]
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburg posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]
- Walker WS, Carnochan F, Pugh G. Thoracoscopic pulmonary lobectomy: early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Hansen HJ, Varela G, Petersen R, et al. Does the number of incisions in video-assisted thoracoscopic surgery matter? J Thorac Dis 2016;8:E1625-7. [Crossref] [PubMed]
- Decaluwé H. One, two, three or four ports does it matter? Priorities in lung cancer surgery. J Thorac Dis 2016;8:E1704-8. [Crossref] [PubMed]
- Craig SR, Walker W. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4. [PubMed]
- Samejima J, Mun M, Matsuura Y, et al. Thoracoscopic anterior 'fissure first' technique for left lung cancer with an incomplete fissure. J Thorac Dis 2016;8:3105-11. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Welcker K, Kesieme E, Intermullo E, et al. Ergonomics in thoracoscopic surgery: results of a survey among thoracic surgeons. Interact Cardiovasc Thorac Surg 2012;15:197-200. [Crossref] [PubMed]
- Licht PB, Ladegaard L. Flexible Thoracoscopy may Facilitate Video-Assisted Thoracoscopic Lobectomy. World J Surg 2010;34:1470-4. [Crossref] [PubMed]
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Example of mechanical scope holder used in our department. Asvide 2017;4:273. Available online: http://www.asvide.com/articles/1582
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Dedicated thoracoscopic instruments allow performing a sharp dissection. Asvide 2017;4:274. Available online: http://www.asvide.com/articles/1583
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Example of exposure achieved by use of throw-off lung forceps. Asvide 2017;4:275. Available online: http://www.asvide.com/articles/1584
- Gossot D, Pryschepau M, Martinez Berenys C, et al. Throw-off instruments for advanced thoracoscopic procedures. Interact Cardiovasc Thorac Surg 2010;10:159-60. [Crossref] [PubMed]
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Opening of a tiny and almost complete fissure on the left side, using a vessel sealing device. Asvide 2017;4:276. Available online: http://www.asvide.com/articles/1585
- Tanaka K, Hagiwara M, Kondo Y, et al. Usefulness of ultrasonically activated scalpel for pulmonary resection in video-assisted thoracoscopic surgery. Kyobu Geka 2006;59:1171-5. [PubMed]
- Santini M, Vicidomini G, Baldi A, et al. Use of an electrothermal bipolar tissue sealing system in lung surgery. Eur J Cardiothorac Surg 2006;29:226-30. [Crossref] [PubMed]
- Tsunezuka Y, Waseda R, Yachi T. Electrothermal bipolar vessel sealing device LigaSureVTM for pulmonary artery ligation – burst pressure and clinical experiences in complete video-assisted thoracoscopic major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2010;11:229-33. [Crossref] [PubMed]
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Temporary control of an intraoperative hemorrhage by packing. Asvide 2017;4:277. Available online: http://www.asvide.com/articles/1586
- Seguin-Givelet A, Traibi A, Grigoroiu M, et al. Example of vascular control using a throw-off bulldog clamp. Asvide 2017;4:278. Available online: http://www.asvide.com/articles/1587
Cite this article as: Seguin-Givelet A, Traibi A, Grigoroiu M, Brian E, Gossot D. Full thoracoscopic fissure based technique for major pulmonary resections: rational and basic considerations. Video-assist Thorac Surg 2017;2:39.