
Left-sided video-assisted thoracic surgery thymectomy
Introduction
The couple thymus and myasthenia gravis (MG) represents one of the most fascinating histories of the medicine as well as of the thoracic surgery. Despite the lack of unequivocal biological links between the thymic gland and MG, the surgical ablation of the thymus has become over the years an integrated part of the treatment of the disease. Thus, it has grown as one of the most fruitful chapter of the thoracic surgery.
After the initial median sternotomy adopted by Blalock (1) for his very first thymectomized patients, several other routes have been progressively proposed (2-4). All of them aimed at lesser invasiveness thus reducing morbidity, acute and chronic pain, infections and esthetic damages related to either total or partial sternotomy. The approaches for total thymectomy have been quite numerous and often combined each other with many technical variances. Regardless the transcervical route, that can be reasonably considered as the very first mini-invasive access, video-assisted thoracic surgery (VATS) proved the preferred and most diffuse approach for extended or maximal thymectomy. This approach was firstly reported by Sugarbaker from Boston (5) and also by the Belgium group (6). This new technique minimized surgical trauma and improved outcome acceptance particularly by young women who occupy a large proportion of MG patient. Initially, the operations were accomplished for non-thymomatous MG, which represents about three quarters of the MG patients (7). With the increment of technical advances and expertise, VATS was also extended to the treatment of early stage thymomas (8).
VATS can be considered the natural evolution of the transcervical route overcoming the most important cons of the open access such as the postoperative pain, the surgical chest trauma and the cosmetic impact. The procedure rapidly gained a wide acceptance and also attracted many young surgeons due to surgical challenge greater than open access (9). However, surgical trainees should be exposed to this kind of surgery only after having become familiar with open access. This basilar caveat minimizes the risks and facilitates the resolution of incidental complications.
Since 1993 we have been routinely performing extended VATS thymectomy through the left sided approach (10). Hereby we focused anatomy, preoperative program, intraoperative technical aspects, postoperative management and commented major controversies.
Anatomy at glance
The aim of thymectomy is to remove as much thymic tissue as possible in order to achieve improvement or remission of MG and prevent tumor spread. Prerequisite to perform a sure VATS thymectomy is based on the robust knowledge of neck anatomy, thoracic inlet and anterior mediastinum. Besides it is very important to have consolidated experience of transsternal thymectomy with at least 20 procedures carried out as first or second surgeon. Thymus is sited approximately in midline in the anterior-superior portion of the mediastinum. During puberty, the thymus weights about 30 to 40 grams and in adult is decreases up to 20–28 grams (7). The gland in the adult appears as a lobulated structure including fat and glandular tissue. With the subsequent physiologic process of involution, the thymic parenchyma is gradually substituted by fibro-adipose tissue. Although variations in size, shape or extent are possible, the organ commonly is formed by the central fusion of two elongated and capsulated lobes assuming an asymmetric H-shaped morphology with two bilateral projecting extremities called superior and inferior horns. Usually, the horns are asymmetric; the right appears larger than the left, which is longer. The superior horns are thinner than the inferior ones. They often reach the cervical area lying deep to sternothyroid muscle. Furthermore, they may lie in close proximity to the recurrent laryngeal nerves.
However, its exact shape is largely molded by the adjacent structures. Anteriorly the thymus is related to the sternum, adjacent to the upper fourth costal cartilage and the sternothyroid and sternohyoid muscles. Posteriorly, it is related to the pericardium, the aortic arch and its branches, the left innominate vein, the trachea and sometimes the thyroid gland. Laterally it relates with the mediastinal pleura.
The thymus is connected with the thyroids gland by two thyro-thymic ligaments. Many variations in the regional anatomy of the thymus are observed. It may lie posterior or anterior to the left innominate vein, and the superior pole of the gland may extend along the pretracheal fascia into the neck area. Laterally, there is a fine capsule that separates it from the pleura and the parapleura mediastinal fat that lies proximal to the phrenic nerves. In the majority of the cases, there is a distinct and recognizable thymic capsule that allows its separation from surrounding mediastinal and cervical structures as well. Also, the horns are easily separable with smooth dissection.
The arterial supply to the thymus originates from the internal thoracic arteries via their pericardio-phrenic branches, and the inferior thyroid arteries. The small arteries enter dorsally through the capsule. Venous drainage is through one or two large veins called the great veins of Keynes that drain into the anterior aspect of the left innominate vein (11). When the thymus lies posterior to the left innominate vein, thymic veins may drain posteriorly. Furthermore, small veins may drain into the inferior and superior thyroid veins and the internal thoracic vein.
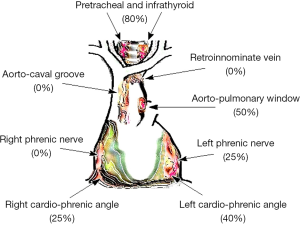
In order to accomplish a true complete thymectomy, it is necessary to take into mind that ectopic foci existing spreads into the mediastinal fat. They can be found in 40% to 70% of the perithymic fat tissue resected during thymectomy (12-14). Jaretzki and Wolf found mediastinal ectopic thymic tissue in 98% of thymectomy specimen, whereas in the cervical area this percentage decreases to 32% (15). They can be prevalently individuated in some areas of the mediastinum whose ablation is pivotal to achieve the best neurological outcome after thymectomy for MG (16). According to our experience we use to identify a number of mediastinal districts where the detection of ectopic foci is more frequent (Figure 1) (17) that should be routinely removed as a part a dedicated post-thymectomy surgical step (18) or during completion procedures for persisting MG despite thymectomy (19).
Preoperative program
Selection
An accurate patient selection is vital to reduce postoperative complications. To this purpose a dedicated multidisciplinary MG team including surgeons, neurologists, pneumologists, intensive care physicians, and physiotherapists is fundamental. Nowadays, VATS thymectomy is offered to young patients of both sex with non-thymomatous MG and small thymomas. Once fully informed the patient accepts more promptly VATS thymectomy.
There is no consensus about the lower and upper age limits, but the procedure is commonly recommended in patients between age 10 and 50 (20,21). Generally, better surgical results are achieved when symptoms have had a relatively recent onset. Uncertainty persists about the role of thymectomy for myasthenic with purely ocular symptoms and those with late onset of the disease. However, it is described that 30–70% of the patients with pure ocular symptoms progress towards generalized myasthenia (22). To date, we successfully perform thymectomy in this subset of patients (23). Between age 5 and 10, the thymectomy is debated and at present there are no clear guidelines. Thymectomy may be mostly considered for children with a markedly severe MG presentation (24).
Patients over 60–65 years should be excluded from the operation, because there is no evidence that the procedure may have any benefit in this subset. Anyway, age should not represent by itself an absolute contraindication to thymectomy. Recently we successfully performed the operation even in elderly patients (≥55 years) suffering with long-standing and refractory MG (25).
Preoperative assessment based on the MG Foundation of America (MGFA) (26) as well as MG quantitative score (27) are very important in evaluating the operative risk.
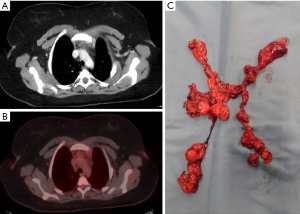
Diagnostic imaging is mandatory in evidencing both the presence and the type of thymic lesions. X-ray imaging, computed tomography (CT) scan, magnetic resonance (MR) imaging and more recently positron emission tomography (PET) (28) provide indispensable information to drive the surgical procedure (Figure 2). A multidisciplinary evaluation of the imaging is important to better choose which side to approach. Indeed, the evidence at imaging of left pleural adhesions or the presence of a prevalently right-sided thymoma may orientate towards a right-sided VATS thymectomy (29).
A previous thyroid surgery or tracheotomy are relative contraindication to VATS thymectomy whereas severe coagulopathy, poor respiratory function, severe underlying pulmonary disease, tenacious bilateral pleural thickening or postoperative adhesions are major contraindications. Finally, imaging of neoplastic invasion of great vessels is an absolute contraindication for minimally invasive thymectomy.
Preparation
As indicated by the American Academy of Neurology the goal of thymectomy is the remission or improvement of symptoms (30). Cooperation with neurologist and physicians is vital. Preoperative management includes anticholinesterases, immunosuppressants, intravenous immunoglobulin and plasmapheresis. A correct timing for the procedure is a pivotal step for patients scheduled for VATS thymectomy. Preoperatively patient should be medically stable especially those having suffered from recent myasthenic crisis. Pharmacologic therapy must be accordingly modified or integrated with intravenous administration of immunoglobulin or plasmapheresis.
Many myasthenic patients may develop postoperative respiratory failure or aspiration pneumonia. Anticholinesterase drugs enhance vagal tone, thus generating to oral hypersecretion and laryngospasm. Besides, prolonged immunosuppressive or steroid therapy might provoke dangerous postoperative infections and wound healing problems.
Our multidisciplinary MG Unit pursues the following tasks: (I) assessment of the neuromuscular symptoms severity; (II) optimization of the pharmacological treatment achieving the stabilization of the symptoms with the lowest anticholinesterases dosage as possible; (III) evaluation of the risk of myasthenic postoperative crisis and preoperative administration of intravenous immunoglobulin and/or plasmapheresis; (IV) evaluation of the risk of respiratory failure and aspiration pneumonia and its prevention with tailored preoperative rehabilitation; (V) estimation of nutritional status and incidental correction with appropriate nutritional support.
Preoperative documentation of respiratory failure may suggest admission to the intensive care unit for ventilator support. All myasthenic patients must be warned and release informed consent about the possibility of postoperative mechanical ventilation. Whatever the approach, thymectomy must be scheduled as the first case in the morning. Appropriate premedication is done avoiding respiratory depressant drugs.
Setting
A perfect position of the patient is extremely important in order to perform a safe and easy VATS thymectomy. Every surgical device should be foreseen and immediately accessible in the operating room before initiating the procedure. An accurate check of the instruments would be advisable. In particular, the risk of emergency shift to an open approach should be considered and therefore the devices to accomplish a fast-track median sternotomy should be rapidly available.
To optimize every surgical phase, the operation should be performed by the trained and rehearsed staff. In our opinion, this is very important to reach optimal surgical results and do not obstacle young trainees from learning.
Usually the setup includes the anesthetic unit, one or better two videothoracoscopic units (composed by a high-definition TV monitor, a video-recorder, 2 light sources, and a video image printer), classic electrocautery and modern energy generating devices. Among the last devices, harmonic (Ultracision®) or radiofrequency (Ligasure®) scalpels are progressively becoming indispensable in the armamentarium. However, also dedicated mechanical instruments have been recently developed allowing most complicated intrathoracic maneuvers and torsion. In particular endoscopic scissors, grasper, swab, hemostatic endoclip applier with specific angular shaft and multiple articulations have been specifically projected and introduced. Nevertheless, the basic traditional instruments should be available, including sponge holding forceps, dental pledget mounted on a curved clamp, and various angulated vascular clamps.
Habitually the 30-degree thoracoscope is sufficient to perform even the most complicated thymectomy. However, more recently, 3-dimension thoracoscope has been introduced in order to allow better planes detection in the whole mediastinal and lower cervical regions.
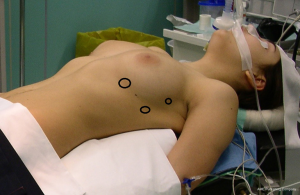
The better exposure of the anterior mediastinum will facilitate all videothoracoscopic maneuvers (31). Thus, the patient should preferentially lie in the semi-supine position, with 20° to 30° rising of the operated hemithorax (Figure 3). A small roll pad is useful to achieve this purpose, while the back rotation of the table permits the widening of intercostal spaces. The arm homolateral to the operated side may be hosted on an arm board rather than outstretched. This position is suitable for both conventional multiport or uniportal accesses. In the case of bleeding, this setting will permit a rapid conversion to open access.
Anesthesia
The anesthetic program should be always planned with the anesthesiologists. Over the years our multidisciplinary group has developed a dedicated anesthetic protocol for VATS thymectomy in myasthenic patients (18,25). The anesthesiologist should have a robust experience in this setting and he must personally visit and evaluate the risk during preoperative assessment in outpatient setting.
As usual, patients have basal parameters monitored including arterial and venous lines, electrocardiogram, non-invasive blood pressure, pulse oxymetry, tidal capnometry, airways pressure, ventilator volume and fraction of inspired oxygen. Monitoring of the neuromuscular transmission with train of four device is obligatory. Inhaled anesthetics can determine satisfactory muscular relaxation (32). If muscle relaxation is required during anesthesia, a reduced dose of intermediate-acting nondepolarizing muscle relaxant can be intravenously infused. Depolarizing neuromuscular blocker agents are not allowed. A nasogastric tube allows the oral administration of anticholinesterase drugs in the early postoperative period.
We operate through selective one-lung ventilation to exclude the required lung. We used a right sided double lumen endobronchial tube. Other methods of one-lung ventilation may be alternatively used when necessary. Thus, a set of double lumen tubes of different caliber must be kept to fit variability in tracheal diameter. Correct positioning of the endobronchial tube is visualized by fiberoptic bronchoscope at the intubation and reconfirmed after patient positioning. Before induction a single stress dose of corticosteroid is given. Induction can be achieved with 2 mg/kg propofol and 2 mcg/kg fentanyl. Usually intubation is performed without the use of muscle relaxant. Inhaled anesthesia with isoflurane 1–2% and 60% nitric oxide in oxygen with single bolus of morphine (0.1 mg/kg) are used along the procedure. Positive end expiratory pressure may be helpful to maintain stable oxygen saturation. To achieve normocapnia, ventilation frequency as well as volumes are adequately controlled. All these actions are done to allow earliest extubation after surgery with the fastest reprise of the spontaneous breathing.
Surgery
Premise
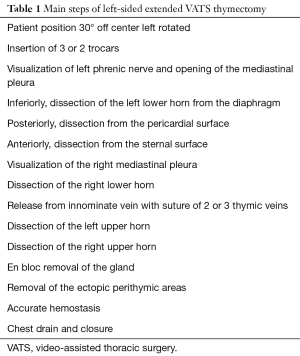
In 1993, we performed the first left-sided extended VATS thymectomy. Since then unilateral VATS has been used with increasing frequency for thymectomy in patients with non-thymomatous MG and both in benign and malignant selected thymic tumors. We experienced that this approach can be done with great safety and excellent intraoperative outcomes. Our initial surgical technique has been described elsewhere (10). It had some evolution over the years, but the principles have remained the same. The procedure may be schematized into fifteen separated steps described in the following Table 1.
Full table
A precise sequence of rules may allow the better execution of the procedure. Prior to port incision we reconfirm the selective ventilation of the right lung. We do not use carbon dioxide insufflation because of the potential adverse effect on patient hemodynamics. Anyway, in order to facilitate cervical, vascular and mediastinal dissection we used to induce a pneumomediastinum 12–24 hours before surgery with 400–600 mL of air insufflated in the supra-jugular notch by a sterile syringe at a rate of 25 mL/min (10). No complications have been observed related to this maneuver.
More important, thymic anatomy largely varies especially in the number of the thymic veins as well as in the place of the thymic horns, which can prolong underneath the innominate vein or laterally the left phrenic nerve or inside the aortopulmonary window. The awareness of these variations ensures a complete resection avoiding severe damages of vascular structures and saving the integrity of the phrenic and recurrent nerves.
The ports of access should be managed aiming at minimizing the chest wall trauma and at reducing postoperative pain. The instruments should be always introduced through a trocar avoiding impingement along the chest wall or a knitting effect inside the pleural cavity. After placement of the first trocar, the subsequent one should be placed under direct vision. The thoracoscope should be never over-twisted. The more anterior ports are preferably used to perform some maneuvers and to extract the surgical specimen because the intercostal space is wider.
The surgical field should be as extended as possible including all thoracic and cervical surfaces thus allowing rapid access to sternotomy.
We prefer left-sided approach for non-thymomatous MG thymectomy and non-invasive thymomas deboarding in the left hemithorax. At present, we use 3 trocars from different diameters (Figure 3). A 10 mm 30° thoracoscope with a high definition camera head and stack is inserted through the first 12 mm trocar camera port under direct cut in front of the tip of the scapula along the midaxillary line in the fourth-fifth left intercostal space.
Subsequently another two 5–10 mm trocars working ports are positioned under direct thoracoscopic vision, one at the second-third intercostal space in the midaxillary line, and the other in the fifth-sixth intercostal space in the anterior axillary line. Whenever necessary a supplementary fourth port may be added at the sixth intercostal space in the middle to anterior axillary line in order to retract the collapsed lung, or enhance the dissection of a huge thymus or exuberant mediastinal fat tissue. Alternatively, a 2-port approach with camera port and working port opened with a soft tissue retractor. This protects the wound edge and allows the use of the suction without re-inflating the lung.
In general, during VATS thymectomy the whole anterior mediastinum is best visualized from the mid to the posterior axillary line. We suggested that the respect the Landreneau’s rule about the trocar arrangement and triangular view with the scope at the apex and the instruments at the base is important for the correct execution of all movements (Figure 3).
Mediastinal dissection
Firstly, the pleural cavity, the anterior mediastinal and the lung are carefully visualized in toto (Figure 4). The left phrenic nerve and incidental pleural adhesions are fully localized. The innominate vein up to the aortic-caval groove is recognized. All these visual informations are vital to predict the feasibility of the VATS thymectomy.
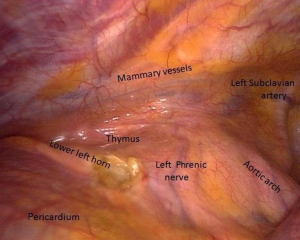
Before initiating mediastinal dissection, a further collapse of the lung through mechanical compression by a sponge holding forceps may be necessary in order to widen the visual field. Pleural adhesions are eliminated by both blunt and sharp dissection. Obviously, if the thymus looks hardly stack to the adjacent structures we must not falter to shift to a transsternal access. We usually proceed according to a precise order of dissection: left lower horn, right lower horn, thymic veins, left upper horn and right upper horn.
After having identified the left phrenic nerve, the mediastinal pleura is opened longitudinally just above the nerve. This incision is extended from the level of the innominate vein up to the limit of the inferior horn of the thymus. Having doing that the sharp dissection of the gland starts inferiorly near the hemidiaphragm and includes the en bloc dissection of all anterior mediastinal tissue and the pericardial fat of the pericardio-phrenic angle. We use an endosgrasp together with endoscissor with electrocautery or more recently with an energy deliver device. Using this tool such as the Ligasure® speed up surgery since blunt dissection, hemostasis and tissue division are feasible without changing instruments.
The gland dissection follows the initial tear of the mediastinal pleura along the anterior margin of the phrenic nerve at the most inferior portion the anterior mediastinal tissue (Figure 5). This dissection proceeds from caudal to cephalad, thus ensuring the identification of phrenic nerve along its full track. Great care is taken not to tear the thymic capsule. Lastly, the dissection is completed anteriorly along the inner surface of the sternum taking care of avoiding the injury of left mammary vessels.
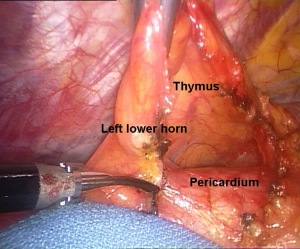
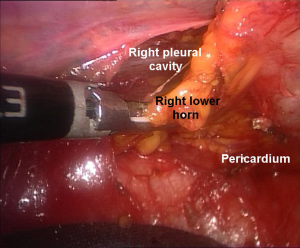
After doing that the left inferior horn of the thymus is entirely identified draping over the pericardium. Next, the sharp dissection continues anteriorly in the retrosternal area following internal thoracic vessels and reaching the contralateral mediastinal pleura. At this point we can discover the right lower horn, which is gently and fully dissected from the pericardium up to the recognized isthmus (Figure 6). We identify the right mediastinal pleura by blunt technique taking care not to enter the pleural cavity. The tissue must include the right pericardiophrenic fat pad up to the right phrenic nerve, which is not visible in all instances. At this step, a particular care deserves the arterial supplies arising from the right mammary internal artery. These branches must be divided by endoclips or Ligasure® devices. The superior vena cava is then cleared of all mediastinal tissue.
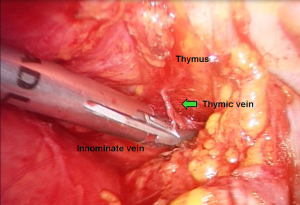
Vascular dissection
Both inferior horns are now mobilized and lifted upward and laterally by blunt dissection to visualize the innominate vein from the origin up to its confluence with the superior vena cava. Total preparation of the vein is carefully performed by the help of pledgets and allows better identification of the thymic venous tributaries, which are variable in number. There are generally two principal branches draining into the inferior aspect of the innominate vein. They are carefully dissected free and closed with 5 mm hemolock® or normal metallic clips prior to dividing as usual (Figures 7,8).
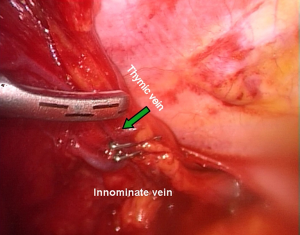
This is always the riskiest step of the procedure and it requires great patience and absolute accuracy. The lesion of these vascular structures is lurking and may oblige to a rapid conversion to an open access.
The accomplishment of vascular step allows the further dissection of the gland. All the further maneuvers are particularly delicate and are facilitated by synergic action with dedicated instruments and mounted pledgets alternating by up or downward traction of the already dissected thymus. All these maneuvers are advantaged by changing of the operative table as by the assistant cooperation in the movements of the thoracoscope.
At this point, thymic lobes tend to fuse and the thymus assumes its typical asymmetric H-shaped morphology. The phrenic nerve, pericardial diaphragmatic vessels and the recurrent laryngeal nerve at the lower edge of the aortic arc must be carefully avoided. The presence of abnormal course of the gland passing behind the innominate vein and the posterior position of the thymic veins requires a careful attention during the dissection.
Cervical dissection
With the thymic gland sufficiently mobile dissection is turned toward the upper horns of the thymus mostly sited in the cervical area. This phase is more exacting than the mediastinal one. The 30° scope provides adequate exposure of this area and confirms the right postero-lateral limit of the dissection, near the right phrenic nerve. When necessary, the instrument port may be changed. The horns appear well encapsulated and differentiated from the surrounding fat by their peculiar pinkish color. The dissection is performed in an extracapsular plan and the entire upper horns are defined to their superior margin so that no thymus residual is left. To this purpose a gentle traction is done to facilitate the releasing of the lower portion of the thymus; the superior horns can be carefully isolated free from the fascial attachment alternatively using the endograsper and blunt dissection on mounted pledget. In addition, patient’s head may be flexed by the anesthesiologist so as the gland can be more easily dissected free from cervical attachment. Small vessels of each superior pole are endoclipped and divided. First, the left upper horn is dissected free. The right upper horn requires a more carefully dissection in freeing it from the cervical bed (Figure 9).
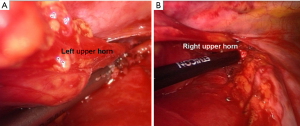
Occasionally at this level anatomical variations in the position of the left upper horn should occur and have to be looked for before extracting the specimen.
Then, entire gland together with the surrounding fat as a free en bloc specimen can be removed using a retriever bag via the most anterior port. An accurate examination of the extracted gland ascertains the integrity of the capsule and its morphology, which can be very variable (Figure 10).
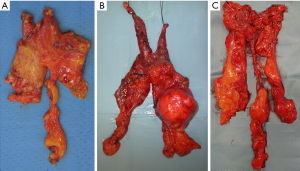
The final step of the procedure entails the resection of the perithymic fat tissue located in the area already described in Figure 1 in order to achieve an anatomically extended thymectomy as required by Masaoka et al. (33) such as anterior mediastinum exenteration from neck to diaphragm and from hilum to hilum.
Difficult cervical dissection should not delay the execution of an adjunctive curved cervicotomy two fingerbreadths above the suprasternal notch. This maneuver facilitates the dissection of the perithyroidal area and maximized the resection of the perithymic cervical fat tissue.
Whenever indicated by the preoperative imaging studies or by the limited anatomy of the neck, we used to perform a priori this cervicotomy. This incision enables us to dissect free the superior thymic horns as well as to enlarge also the surgical field during mediastinal VATS steps. In addition, the cervicotomy permits the silk ligature of the thymic veins, avoiding the use of hemoclips that have the tendency to slip off during the subsequent VATS dissection with a resulting troublesome bleeding.
At the end, the whole thymic bed is carefully inspected for hemostasis and completeness of resection especially in patients who underwent plasmapheresis. Particular care should be done to the skeletonized main venous axis. The insertion of a single #22–28 French chest drain through the lower port site, positioned under vision in the mediastinal area. is opportune. It is a good rule to observe the optimal re-expansion of the inflated lung before layered closure of the wound ports. The drain is secured with a #1 silk suture. If the contralateral pleura has been opened the tip is placed through the defect to drain both pleural cavity.
Complications
Blunt dissection of the innominate vein axis remains one of the most important step in VATS thymectomy. Superior vena cava and aortic injuries are less frequent (34). This problem is potentially life-threatening and can more easily occur in presence of local adhesions and in obese patients.
A small injury may be amenable to the topical hemostatic by thoracoscopic cotton swab and pressure applied through the most convenient port, thus avoiding sternotomy. The use Tacoseal® or other hemostatic device may be useful for definitive hemostasis.
Conversely, a larger injury should be initially handled by having the area packed with the assistant applying pressure with a rolled-up sponge. During bleeding an additional port can be created in the mid-clavicular line at the fifth-sixth intercostal space to obtain better pressure. If pressure hemostasis is not sufficient sternotomy must be immediately performed. The conversion to sternotomy must not be felt as a surgical failure even in the most experienced VATS surgeons. Once again, we emphasize that sternotomy should be among the essential surgical capacities of a surgeon facing a VATS thymectomy.
Postoperative care
In the well preoperatively prepared patient, the postoperative care is straightforward and likely uncomplicated. Extubation should be hastened after surgery. After a brief staying in the recovery room, the admission in the surgical ward is routine after anesthesiological evaluation. We prefer that the same anesthesiologist has to look after the patient up to the return in the ward. A sitting chest X-ray is performed within one hour after surgery to ensure complete re-expansion of the lung and exclude hemothorax. If no air leak or bleeding occur through the chest drainage, this can be removed in the recovery room 2–4 hours after surgery.
Respiratory function is early monitorized at the bedside both in recovery and at ward. If there are severely altered volumes, appropriate medical care is instituted. Signs of fatigue and respiratory failure should be associated with progressive weakness should induce the anesthesiologists to restore ventilator support.
At the same the neurologist timely evaluates the clinical evolution of the myasthenic symptoms. Anticholinesterases and steroids are conveniently administered by nasogastric tube in the recovery room or as soon as possible by mouth. The patient can resume a full diet when fully awake from the general anesthesia unless in presence of bulbar symptoms. Postoperative pain is adequately controlled by standard oral analgesics.
Respiratory and muscle rehabilitation program under adequate pharmacological coverage is started immediately after the operation (35). When required, swallowing and speech rehabilitation therapists may be also involved.
Patients are usually discharged within 3 days after VATS, whereas postoperative generalized MG may require a longer stay.
The first follow up is done within 3–4 weeks after surgery with a multidisciplinary MG evaluation.
Comment
To date, thymectomy is everywhere accepted as a valid treatment for MG (21). For decades, median sternotomy and its variables dominated the surgical treatment of MG and thymomas. However, high morbidity, persistent pain and poor cosmesis, have stimulated the research of a lesser traumatic pathway but with similar benefits. Transcervical thymectomy represents the first mini-invasive approach with success used by many surgeons (36-39).
The advent of VATS in the scenario of thoracic surgery has promoted its employ in thymectomy, firstly for non-thymomatous MG and thereafter for selected thymomas (40). The procedure has soon presented evident advantages in particular when matched with the inconveniences arising from median sternotomy.
Indeed, reduced surgical trauma with lesser acute and chronic pain, less morbidity, shorter hospital stay, safeguard of the cosmesis, immediate return of the day-life and occupational activities are the most relevant achievements featured by VATS thymectomy (9).
All these merits highly increased the confidence of both myasthenic patients and neurologists who are becoming less reluctant towards early surgical treatment. An increasing number of surgeons prefers now VATS thymectomy given the superb visualization of the mediastinum provided by new technologies and with the introduction of novel instruments. VATS technique has significantly evolved over the years allowing thymectomy through uniportal (41) or subxiphoid (42,43) accesses and under non-intubated anesthesia (44).
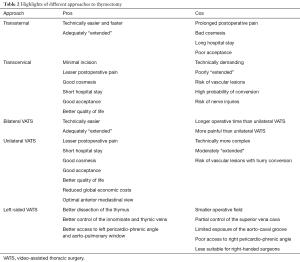
Although randomized, prospective clinical investigations are still missing, the analysis of the available data confirms that VATS thymectomy is presently a safe procedure with unquestionable immediate advantages (45-52) (Table 2) involving both non-thymomatous MG and selected thymomas (53). VATS usually results with lower operative blood loss and thoracic drain volumes (54). Longer operative time documented at the beginning of the experience (55), has been significantly reduced (56). Rückert et al. demonstrated preserved pulmonary function in the immediate postoperative period after VATS operation (57); this condition leads to less pulmonary infection with a relevant importance in myasthenic patients. Finally, initial elevated costs of VATS thymectomy can be counterbalanced by the decrement of postoperative expenses due to significant shorter in-hospital time length. This last advantage makes VATS thymectomy more profitable even in developing countries as outlined by Chicaiza-Becerra and colleagues (58).
Full table
VATS thymectomy has shown to provide better or at least similar quality of life and long-term results compared to open surgery (59). A recent meta-analysis from Gung et al. (60) based on a total of 23 trials regarding VATS Vs sternotomy in non-thymomatous MG evidences significant lower morbidity rate and shorter hospital stay with similar long-term results.
Another meta-analysis from Friedant et al. (56) compares the two approaches on thymic malignancies in 30 different studies. All advantages of VATS thymectomy are confirmed and no significant differences in rate of R0 resections and overall recurrence rate were detected.
VATS approach includes bilateral and unilateral, right or left-sided VATS. Extended thymectomy was feasible by either side. In non-thymomatous MG a controversy about the exact technique and better side to approach still persists (61). Visualization of the innominate vein and its branches may represent the only relevant difference between the two procedures. The right-sided VATS demonstrates that the innominate vein may be easier dissected along the superior vena cava. From the left side, this maneuver is more tedious because the superior vena cava lies outside from the surgical field. In 1995, Yim et al. (62) proposed that a right-side approach could be appropriate for VATS. This side allows larger operating space with an easier visualization of the innominate vein, and wider possibility of removing the mediastinal fat sited right pericardio-phrenic angle and aorto-caval groove.
We prefer the left-sided route because the left side of the thymus gland is usually larger extending down to pericardio-phrenic area. Thus, this approach permits an extensive removal of fat sited in aorto-pulmonary window and left pericardio-phrenic angle that we found more often location of ectopic thymic tissue (25). Rückert et al. in an anatomical study have demonstrated that the left approach may remove more tissue that the right one (63). The left-sided VATS results mostly preferred in the case of thymomas, which are more often left-off center located (64). Although our personal experience in VATS for thymomas is limited, the vast majority of the procedure was successfully executed through the left side.
In general, significant differences were not detected between both sided-accesses when comparing surgical time, intraoperative blood loss, postoperative complications, hospital stay and thoracic drain volumes (65). These considerations induce to consider VATS thymectomy successfully amenable from either side. Hence the choice of the side should only depend on personal experience and by the prevalent location of the thymus and thymoma, and by local condition predicted by imaging.
An assortment of approaches is now offered to the surgeons since the very beginning of their experience. In making their choice they cannot neglect the knowledge of the median sternotomy, which remains the cornerstone of the thymic surgery. When accomplishing a unilateral VATS thymectomy they can choose the side to approach according not only to their preference and skill, but also according to imaging findings. Non-thymomatous MG and thymoma can be adequately treated by VATS (66).
Conclusions
Video-assisted thymectomy was introduced in early ‘90s as a minimally alternative technique for patients with non-thymomatous MG. Less morbidity and greater patient acceptance favored its diffusion and appreciation. Thus, earlier thymectomies became more common in patients with non-thymomatous MG. To date VATS thymectomy is worldwide accepted as an effective and safe treatment for MG and early thymomas. Advocates of VATS thymectomy insist that this minimal approach is associated with less tissue trauma, lower complication rate, reduced acute and chronic postoperative pain and hospital stay. Furthermore, respect of the thoracic cosmesis is particularly emphasized by the young myasthenic women. VATS approaches include unilateral, either left- or right-sided, or bilateral. Each side-approach presents advantages and limits. This technique can be safely and successfully performed in the either side of the thorax by experienced surgeons, which must have a solid experience in both mediastinal surgery and open thymectomy. Twenty-five years after its very first appearance and having used all proposed routes to thymectomy (67,68), we can affirm that VATS thymectomy has stood the test of the time, which is the only that definitively establishes the validity of a surgical procedure. However, the ideal approach to thymus continues to be a challenging surgical problem.
Acknowledgments
We would like to thank all the staff members of the Myasthenia Gravis Unit at the Tor Vergata University including neurologists, anesthesiologists, intensivists, psychologists, physiotherapists and nurses.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Minimally invasive VATS thymectomy for Myasthenia Gravis” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.05.05). The series “Minimally invasive VATS thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region. Report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Blalock A. Thymectomy in the treatment of myasthenia gravis: report of twenty cases. J Thorac Cardiovasc Surg 1944;13:316-39.
- Kirschner PA, Osserman KE, Kark AE. Studies in myasthenia gravis. Transcervical total thymectomy. JAMA 1969;209:906-10. [Crossref] [PubMed]
- Miller JI, Mansour KA, Hatcher CR Jr. Median sternotomy T incision for thymectomy in myasthenia gravis. Ann Thorac Surg 1982;34:473-4. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Coosemans W, Lerut TE, Van Raemdonck DE. Thoracoscopic surgery: the Belgian experience. Ann Thorac Surg 1993;56:721-30. [Crossref] [PubMed]
- Drachman DB. Myasthenia gravis. N Engl J Med 1994;330:1797-810. [Crossref] [PubMed]
- Kaiser LR. Thymoma. The use of minimally invasive resection techniques. Chest Surg Clin N Am 1994;4:185-94. [PubMed]
- Mineo TC, Ambrogi V. Moving around the thymus: More technology, more safety, more efficiency. J Thorac Cardiovasc Surg 2016;152:280-1. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Adjuvant pneumomediastinum in thoracoscopic thymectomy for myasthenia gravis. Ann Thorac Surg 1996;62:1210-2. [Crossref] [PubMed]
- Keynes G. The results of thymectomy in myasthenia gravis. Br Med J 1949;2:611-6. [Crossref] [PubMed]
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5. [Crossref] [PubMed]
- Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-9. [Crossref] [PubMed]
- Klimek-Piotrowska W, Mizia E, Kuzdzal J, et al. Ectopic thymic tissue in the mediastinum: limitations for the operative treatment of myasthenia gravis. Eur J Cardiothorac Surg 2012;42:61-5. [Crossref] [PubMed]
- Jaretzki A 3rd, Wolff M. "Maximal" thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J Thorac Cardiovasc Surg 1988;96:711-6. [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. “Maximal” thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Video-assisted thoracoscopic thymectomy surgery: Tor Vergata experience. Thorac Cardiovasc Surg 2015;63:187-93. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted completion thymectomy in refractory myasthenia gravis. J Thorac Cardiovasc Surg 1998;115:252-4. [Crossref] [PubMed]
- Evoli A. Clinical presentation, diagnosis and classification. In: Mineo TC. editor. Novel challenges in myasthenia gravis. New York: Nova Science Publishers Inc., 2015:171-88.
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141-9. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:1319-24. [Crossref] [PubMed]
- Castro D. Juvenile myasthenia gravis: from diagnosis to treatment. In: Mineo TC. editor. Novel challenges in myasthenia gravis. New York: Nova Science Publishers Inc., 2015:207-22.
- Mineo TC, Ambrogi V. Left VATS thymectomy. In: Mineo TC. editor. Novel challenges in myasthenia gravis. New York: Nova Science Publishers Inc., 2015:415-49.
- Jaretzki A III, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [Crossref] [PubMed]
- Barohn RJ, McIntire D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci 1998;841:769-72. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Schillaci O. May positron emission tomography reveal ectopic or active thymus in preoperative evaluation of non-thymomatous myasthenia gravis? J Cardiothorac Surg 2014;9:146. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V. Video-assisted thoracoscopic thymectomy: from the right or from the left? J Thorac Cardiovasc Surg 1997;114:516-7. [Crossref] [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:7-15. [Crossref] [PubMed]
- Ng JW, Yeung GH, Cheng DP. Video-assisted thymectomy in patients with myasthenia gravis: lateral versus supine position. J Thorac Cardiovasc Surg 1998;115:265-6. [PubMed]
- Pottecher J, Helms O. Innovations in anesthesia for thymectomy in myasthenic patients. In: Mineo TC. editor. Novel challenges in myasthenia gravis. New York: Nova Science Publishers Inc., 2015:341-57.
- Masaoka A, Nagaoka Y, Kotake Y. Distribution of thymic tissue at the anterior mediastinum. Current procedures in thymectomy. J Thorac Cardiovasc Surg 1975;70:747-54. [PubMed]
- Toker A, Ozkan B. Videothoracoscopic thymectomy for myasthenia gravis: an overview of complications on 387 VATS thymectomies for myasthenia gravis. Video-assist Thorac Surg 2017;2:19. [Crossref]
- Ambrogi V, Mineo TC. Benefits of comprehensive rehabilitation therapy in thymectomy for myasthenia gravis: a propensity score matching analysis. Am J Phys Med Rehabil 2017;96:77-83. [Crossref] [PubMed]
- Papatestas AE, Pozner J, Genkins G, et al. Prognosis in occult thymomas in myasthenia gravis following transcervical thymectomy. Arch Surg 1987;122:1352-6. [Crossref] [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Shrager JB, Deeb ME, Mick R, et al. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg 2002;74:320-6; discussion 326-7. [Crossref] [PubMed]
- de Perrot M, Bril V, McRae K, et al. Impact of minimally invasive trans-cervical thymectomy on outcome in patients with myasthenia gravis. Eur J Cardiothorac Surg 2003;24:677-83. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Ng CS, Rocco G, Wong RH, et al. Uniportal and single incision videoassisted thoracic surgery. The state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through and infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Yano M, Moriyama S, Haneda H, et al. The subxiphoid approach leads to less invasive thoracoscopic thymectomy than the lateral approach. World J Surg 2017;41:763-70. [Crossref] [PubMed]
- Jiang L, Liu J, Shao W, et al. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy using glasses-free 3D vision. J Thorac Dis 2016;8:E1602-4. [Crossref] [PubMed]
- Meyer DM, Herbert MA, Sobhani NC, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transternal and minimally invasive approaches. Ann Thorac Surg 2009;87:385-90. [Crossref] [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Lin MW, Chang YL, Huang PM, et al. Thymectomy for non-thymomatous myasthenia gravis: a comparison of surgical methods and analysis of prognostic factors. Eur J Cardiothorac Surg 2010;37:7-12. [Crossref] [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Yang Y, Dong J, Huang Y. Thoracoscopic thymectomy versus open thymectomy for the treatment of thymoma: a meta-analysis. EJSO 2016;42:1720-8. [Crossref] [PubMed]
- Siwachat S, Tantraworasin A, Lapisatepun W, et al. Comparative clinical outcomes after thymectomy for myasthenia gravis: thoracoscopic versus trans-sternal approach. Asian J Surg 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [PubMed]
- Toker A, Erus S, Ziyade S, et al. It is feasible to operate on pathological Masaoka stage I and II thymomas patients with video-assisted thoracoscopy: analysis of factors for a successful resection. Surg Endosc 2013;27:1555-60. [Crossref] [PubMed]
- Chang PC, Chou SH, Kao EL, et al. Bilateral video-assisted thoracoscopic thymectomy vs. extended transsternal thymectomy in myasthenia gravis: a prospective study. Eur Surg Res 2005;37:199-203. [Crossref] [PubMed]
- Shiono H, Kadota Y, Hayashi A, et al. Comparison of outcomes after extended thymectomy for myasthenia gravis: bilateral thoracoscopic approach versus sternotomy. Surg Laparosc Endosc Percutan Tech 2009;19:424-7. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [Crossref] [PubMed]
- Chicaiza-Becerra LA, Garcia-Molina M, Gamboa O, et al. The cost-effectiveness of open or thoracoscopic thymectomy compared to medical treatment in managing Myasthenia gravis without thymomas. Rev Salud Publica (Bogota) 2012;14:260-70. [Crossref] [PubMed]
- Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. [Crossref] [PubMed]
- Gung Y, Zhang H, Li S, et al. Sternotomy versus video-assisted thoracoscopic surgery for thymectomy of myasthenia gravis patients: a meta-analysis. Asian J Endosc Surg 2016;9:285-94. [Crossref] [PubMed]
- Xie X, Gan X, Chen B, et al. Left- and right-sided video-assisted thoracoscopic thymectomy exhibit similar effects on myasthenia gravis. J Thorac Dis 2016;8:124-32. [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [Crossref] [PubMed]
- Rückert JC, Czyzewski D, Pest S, et al. Radicality of thoracoscopic thymectomy. An anatomical study. Eur J Cardiothorac Surg 2000;18:735-6. [Crossref] [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic diseases. World J Surg Oncol 2014;12:398. [Crossref] [PubMed]
- Tomulescu V, Ion V, Kosa A, et al. Thoracoscopic thymectomy mid-term results. Ann Thorac Surg 2006;82:1003-7. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1. [Crossref] [PubMed]
- Mineo TC. Terapia chirurgica della myasthenia gravis. Atti Acad Lancisiana Roma 1978;23:1-12.
- Mineo TC, Cirulli C, Rea S, et al. La via transcervicale nella chirurgia del timo. Sett Osped 1978;20:59-63.
Cite this article as: Mineo TC, Sellitri F, Ambrogi V. Left-sided video-assisted thoracic surgery thymectomy. Video-assist Thorac Surg 2017;2:32.


