
Uniportal video-assisted thoracoscopic thymectomy
Introduction
Thymoma is the most common type of mediastinum tumor, and transsternal thymectomy with complete tumor excision is the standard treatment for patients with thymoma. With advances in endoscopic techniques and equipment, thoracoscopic thymectomy has been widely performed in early stage thymoma patients. Compared with transsternal thymectomy, thoracoscopic thymectomy has been associated with improved shorter hospital stays, lower operative blood loss and decreased postoperative pain (1,2). However, surgeons continue to pursue ever less invasive while effective surgical methods of thymectomy. Single port video assisted thoracoscopic thymectomy is one kind of minimally invasive surgery in use today. Dr. Suda and Dr. Wu have proved its safety and feasibility (3-6). Although such an approach for thymectomy is already feasible and may be preferable, there are several aspects worth exploring further and in-depth, including the criteria of operative indication, operation tricks, pitfalls, postoperative care and so on.
Patient selection and workup
All patients receive physical examinations, blood routine, and neurological assessment before surgery. The most important image examination is computed tomography of the chest with contrast. Chest CT with contrast can help surgeons to verify the feasibility of single port VATS thymectomy. Only patients without invasion of the pericardium or great vessels are suitable to receive single port thymectomy. However, one thing is worth noting that contrast might induce exacerbation of myasthenia gravis-related symptoms, such as new or progressive acute respiratory compromise in MG patients (7).
Pre-operative preparation
Patients are placed in a 30-degree semi-supine position on the operating table with a roll placed beneath the shoulder and the ipsilateral arm held abducted over a padded L-screen in order to expose the axillary area. Split lung ventilation is completed by double-lumen endotracheal intubation. For singe port VATS thymectomy, selection of the entrance side for the operation is decided by the need for safe and complete resection of all thymic tissue. We prefer to perform single port thymectomy from the right side because the surgeon can get a better visualization of the thymic vein and tissue surrounding the thymus. The operator and first assistant (cameraman) stand at the same side. A high resolution camera and monitor are basic facilities and the monitor is arranged in front of the operator and first assistant so that the surgeon can have a direct and unobstructed view of the video image during the operation.
Operation procedure
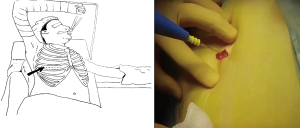
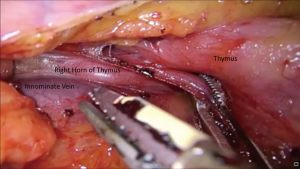
A 2–3 cm wound incision is initiated at the pivot of the 4th intercostal space and anterior intercostal line (Figure 1). After creating the wound, the operator can use the endoscope or a finger to see whether there is pleural symphysis or not. If pleural symphysis exists around the wound, the operator should first delicately perform pneumolysin before thymectomy. When entering the chest wall cavity, the operator can easily discriminate the structures of the thymus, the bifurcation of the vena cava and innominate vein, and the right side phrenic nerve (Figure 2A). Thymectomy is initiated by incision to the mediastinal pleura along the inferior and anterior border of the thymus. Using an energy device, such as harmonic scalpel (Ethicon, USA), with the operator’s dominant hand, while manipulating the suction tube with the non dominant hand, the thymus tissue can be easily detached from the pericardium and moved to the superior border (Figure 2B). Until the confluence of innominate vein and superior vena cava can be seen, the mediastinal pleura at the superior border is incised along the length of the internal mammary artery/vein at the level of the sternum. Then, tracing the innominate vein, the drainage vein of the thymus can easily be found (Figure 3). After dividing the drainage of the thymic vein, the dissection proceeds above the innominate vein and right horn of the thymus. At this moment, gentle traction is applied to the right horn with the grasper downward to dissect the thymic tissue to the lower cervical area (Figure 4). After liberating the right horn of the thymus, we release the left horn with the same method. Sometimes, the surgeon might encounter some supply or drainage vein from the internal mammary artery and vein, and the operator should divide these anonymous vessels carefully. After the whole thymic tissue and surrounding fatty tissue are completely resected, endobag or surgical glove is introduced into the chest wall cavity under thoracoscope and using the grasper the specimen is placed into the endobag or surgical glove without squeezing the capsule of the specimen. Once the whole specimen is totally placed in the endobag or surgical glove, it can be extracted from the chest wall and sent for further pathological examination.

Post-operative management
If the patient can successfully resume spontaneous respiration, we will directly transfer this patient to the ordinary ward. If the patient cannot recover smoothly, especially in cases of Myasthenia Gravis, we transfer them to the intensive care unit until ventilator support is no longer needed. It is also important to consider the impact of pain on patients following single-port surgery. However, pain is subjective and may be affected by a number of factors, including operative time, drainage tube size, the area of intercostal nerve affected and post-operative analgesic regimens. Avoiding the use of chest tubes post thymectomy might also help to reduce postoperative pain, but this can only be considered for simple cases of thymectomy. If the patient suffers from giant thymoma, Myasthenia Gravis, or thymoma severely attached to surrounding tissue, chest tube placement will be necessary. In addition, placement of a subpleural catheter with continual intercostal analgesia is another method to relieve postoperative pain (8).
Tricks and pitfalls
Although this article deals specifically with single port thymectomy, the main principles of surgical technique are the same as those used for most single port surgery. The location of the incision is the rate determining step of the whole surgery. The 4th intercostal space is usually the best choice for the incision. A rubber wound protector can help the newbie to overcome initial incompatibility, but is not suitable for pleural symphysis patients because the rubber wound protector might decrease the space for manipulation. Furthermore, dissection along the right side of the phrenic nerve and innominate vein, and avoiding injury to the left phrenic nerve, especially in Myasthenia Gravis patients, are of primary concern in single port thymectomy. Bleeding accident may occur during dissection of the thymic vein from the innominate vein. In such situations, using the grasper with gauze to compress the bleeding area is the first step to control the bleeding. If the surgeon finds they can’t handle the bleeding, they shouldn’t hesitate to convert to open surgery. Surgeons can shift endoscopic surgery to anterior thoracotomy or sternotomy quickly on account of the patient’s prepared position as mentioned above. Whether anterior thoracotomy or sternotomy is necessary depends on the location of the bleeding point. Sternotomy is suggested if the innominate vein main trunk is injured because sternotomy might provide a better field of operation to control the bleeding.
Discussion
The true significance of minimally invasive surgery is not only to reduce the size of the wound but also to reduce the inner trauma of patients. Several reports have shown that VATS patients have better immune function than open surgery patients (9,10). Technically speaking, single port surgery is the least invasive surgery in the world, and with such minimally invasive surgery, we expect to do less harm to our patients and that patients can recover better. In the near future, research comparing the immune response in multiport VATS and singe port VATS may show whether this new technique can indeed bring less injury to patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Minimally invasive VATS thymectomy for Myasthenia Gravis” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.03.02). The series “Minimally invasive VATS thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. DGR serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jul 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang Y, Dong J, Huang Y. Thoracoscopic thymectomy versus open thymectomy for the treatment of thymoma: A meta-analysis. Eur J Surg Oncol 2016;42:1720-8. [Crossref] [PubMed]
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Comparative Short-Term Clinical Outcomes of Mediastinum Tumor Excision Performed by Conventional VATS and Single-Port VATS: Is It Worthwhile? Medicine (Baltimore) 2015;94:e1975 [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Somashekar DK, Davenport MS, Cohan RH, et al. Effect of intravenous low-osmolality iodinated contrast media on patients with myasthenia gravis. Radiology 2013;267:727-34. [Crossref] [PubMed]
- Wu CF, Hsieh MJ, Liu HP, et al. Management of post-operative pain by placement of an intraoperative intercostal catheter after single port video-assisted thoracoscopic surgery: a propensity-score matched study. J Thorac Dis 2016;8:1087-93. [Crossref] [PubMed]
- Ng CS, Wan IY, Yim AP. Impact of video-assisted thoracoscopic major lung resection on immune function. Asian Cardiovasc Thorac Ann 2009;17:426-32. [Crossref] [PubMed]
- Ng CS, Wan S, Hui CW, et al. Video-assisted thoracic surgery for early stage lung cancer - can short-term immunological advantages improve long-term survival? Ann Thorac Cardiovasc Surg 2006;12:308-12. [PubMed]
Cite this article as: Wu CF, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic thymectomy. Video-assist Thorac Surg 2017;2:25.



