Subxiphoid VATS thymectomy for myasthenia gravis
Introduction
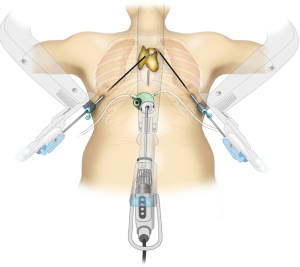
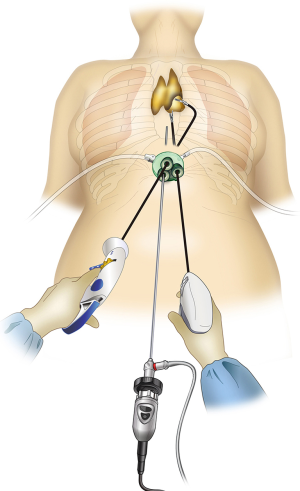
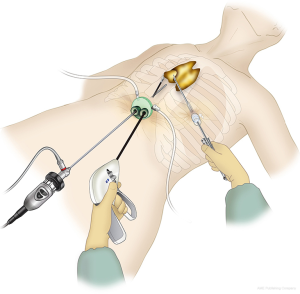
Subxiphoid single-port thymectomy (SPT; Figure 1) is the removal of the thymus via a single 3-cm incision made below the xiphoid process (1). Because the camera is inserted through the midline subxiphoid region, the operative view in the neck region and locations of the bilateral intercostal nerves are easy to verify. Furthermore, because the intercostal space is not traversed, there is no intercostal nerve damage and the pain is minimal (2). The single incision on the abdomen offers an excellent cosmetic outcome. On the other hand, the drawbacks of SPT are poor operability and difficult suturing. For cases that require suturing, subxiphoid dual-port thymectomy (DPT; Figure 2), wherein an additional port is inserted through the lateral intercostal space (3), or subxiphoid robotic thymectomy (SRT; Figure 3) (4) is indicated. Herein we introduce the state of art surgical techniques and initial outcomes of SPT, DPT, and SRT for myasthenia gravis (MG).
Methods
Patient selection
SPT is indicated for MG patients without a thymoma. When suturing is required, such as when pericardial patch closure is required for a thymoma that has infiltrated the pericardium, DPT or SRT, both of which have better operability, is indicated. When suturing is required for tumor infiltration into a blood vessel such as the innominate vein, surgery is usually performed via median sternotomy.
Preoperative surgical preparations
SPT is performed with the laparoscopic instrument (LigaSure™ Maryland; Covidien, Mansfield, MA, USA) held in the right hand and the grasping forceps with a single-port flexible tip (SILS™ Hand Instruments, SILS Clinch, 36 cm; Covidien, Mansfield, MA, USA) held in the left hand. In SPT, it is vital to use grasping forceps with a flexible tip to prevent interference between the forceps. In DPT, there is no need for the single-port flexible tip, and grasping forceps alone, such as the one used in routine thoracoscopic surgery, is acceptable. Due to the shape of its tip, the LigaSure™ Maryland laparoscopic instrument is not only suitable for hemostasis and coagulation incisions but also appropriate for dissection. The port for this surgical procedure should have a single-port design that permits carbon dioxide (CO2) insufflation. To date, we have used many different ports, but we feel that GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA, USA) has the best operability because the port platform is gel-like and child ports are not excessively immobilized. Figure 4 shows the positions of child ports that we use. The camera scope that we use is a 5-mm-wide rigid endoscope with a 30° perspective. In addition, safe surgery would be impossible without a bright monitor screen that projects the camera scope images.

Positioning
The patient is placed in the supine position with the legs apart. The surgeon stands between the patient’s legs, and the surgical assistant stands at the patient’s right side to operate the camera scope. The monitor is positioned above the patient’s head. The range of disinfection is from the subxiphoid region to the neck region in case there is a need to switch to median sternotomy.
Insertion of the port via a subxiphoid incision
The subxiphoid approach has not been performed often, possibly because cardiothoracic surgeons are unfamiliar with this technique. Herein we describe the method of inserting the port via a subxiphoid incision.
A 3-cm longitudinal or transverse skin incision is made approximately 1 cm below the xiphoid process. A transverse incision follows the direction of the Langer’s lines of the skin and can make the postoperative wound less conspicuous, whereas a longitudinal incision has the advantage of facilitating the extension of the skin incision in the event that a larger tumor needs to be removed. With this approach, the author has extracted tumors up to a maximum diameter of 11 cm. After making the skin incision, the location of the xiphoid process is verified by palpation. The adherent rectus abdominis muscle is detached to expose the cartilaginous section of the xiphoid process; there is no need to resect the xiphoid process. A finger is then blindly inserted behind the xiphoid process to detach a sufficient area of the posterior surface of the sternum from the thymus. It is advisable to detach the surface by as much as possible with a finger. A 5-mm incision is made into the fascia of the rectus abdominis, followed by blunt finger dissection to create space inside the fascia for port insertion. During this process, caution is required to avoid tearing the peritoneum. Although surgery can proceed even with a torn peritoneum, the resulting pneumoperitoneum can complicate the procedure. Once the port has been inserted, CO2 insufflation at 8 mmHg is performed to dorsally retract the pericardium and create a narrow, but sufficient, space for surgery. From this point, the thymus is detached from the posterior surface of the sternum in the direction of the neck region. Due to the shape of the thorax, the inflexible tip of the LigaSure™ instrument may not reach the back of the sternum. In such cases, an instrument such as an endoscopic scissors with a flexible tip is used to detach the thymus from the posterior surface of the sternum. As the thymus is detached, pressure from CO2 insufflation gradually causes the space at the back of the sternum to widen.
Subxiphoid single-port thymectomy
The author usually makes bilateral mediastinal pleural incisions to open the thoracic cavity and verify the location of the phrenic nerve. Furthermore, this type of incision dorsally shifts the heart due to gravity to secure a larger surgical space. When a tumor has invaded one side of the thoracic cavity, surgery is sometimes performed without detaching the contralateral mediastinal pleura, if possible, to prevent tumor seeding to the opposite side. First, the adipose tissue is grasped from the pericardium on the left side before detaching it from the pericardium in front of the left phrenic nerve. The thymus and surrounding adipose tissue are detached from the pericardium until the distal end of the innominate vein can be verified. Next, the thymus and surrounding adipose tissue in front of the right phrenic nerve are detached from the pericardium from the caudal end toward the cranial end to expose the central portion of the innominate vein. The superior poles of the thymus and surrounding adipose tissue in the neck region are detached from the right brachiocephalic vein, from the trachea and brachiocephalic artery on the dorsal side, and from the superior margin of the distal end of the innominate vein on the left side. A technique for revealing a good operative view in the neck region is to hold the detached superior poles of the thymus with a grasping forceps and to caudally pull them to dorsally retract the innominate vein. Finally, the thymic vein is cut with a vessel-sealing device, followed by en bloc resection of the thymus and its surrounding adipose tissue. The bag used to collect the resected thymus can be one without a frame because an additional frame can tangentially shift the opening of the bag with the forceps, making it difficult to insert the thymus into the bag. The technique of SPT is presented in Figure 5 (5).

Subxiphoid dual-port thymectomy
DPT is a surgical approach with improved operability through an additional intercostal port on the anterior chest and was originally designed for cases when SPT is difficult (3). Similar to SPT, DPT involves a 3-cm skin incision below the xiphoid process. A port is inserted, and CO2 insufflation is performed to detach the thymus from the back of the sternum, after which bilateral mediastinal pleural incisions are made to open the thoracic cavity. A 5-mm port is then inserted into the fifth intercostal space where the tumor is located. The camera scope is usually inserted via a subxiphoid port, but it can also be inserted via an intercostal port. Using a dual-port system eliminates interference between the forceps and facilitates suturing.
Subxiphoid robotic thymectomy
In 2015, the author and his colleagues reported SRT as a new surgical technique wherein a camera is inserted via a subxiphoid incision and robotic arms are inserted through bilateral intercostal spaces (4).
Similar to SPT and DPT, SRT involves a 3-cm skin incision below the xiphoid process. After making bilateral mediastinal pleural incisions and opening the thoracic cavity on both sides, 1-cm skin incisions are made into the sixth intercostal spaces on the bilateral axillary lines through which the ports for SRT are inserted. Thereafter, a da Vinci Si Surgical System® (Intuitive Surgical, Sunnyvale, CA, USA) is docked from the cranial end. After fitting the da Vinci Si Surgical System®, a da Vinci camera scope is inserted into the subxiphoid port designed for single-port surgery. A spatula connected to a monopolar coagulator or a Maryland forceps connected to a bipolar coagulator is fitted into the right arm of the da Vinci system, while a fenestrated grasping forceps connected to a bipolar coagulator or a Cadiere forceps is fitted into the left arm. In some cases, the surgical assistant pulls out the thymus with a single-port surgical forceps via the single port to expand the surgical field. The thymic vein is cut using an EndoWrist Vessel Sealer (Intuitive Surgical, Sunnyvale, CA, USA). The technique of SRT is presented in Figure 6 (6).

Results
Of 83 patients who underwent thymectomy via the subxiphoid approach at our hospital between March 2011 and June 2016, 15 had MG. The initial outcomes of each approach are presented in Table 1. Data are shown as mean ± standard deviation. MG patients comprised six men and nine women; based on the Myasthenia Gravis Foundation of America classification scale, four were classified as class I, one as class IIa, five as class IIb, three as class IIIa, and two as class IVa. Six patients (40%) had concomitant thymoma; according to the World Health Organization classification for these thymomas, three patients had type AB and three had type B2. Before surgery, 11 patients were indicated for SPT, one was indicated for DPT, and three were indicated for SRT. One patient who underwent SPT also underwent partial pneumonectomy with a stapler for suspected infiltration of the thymoma into the lung. Another patient was switched from SPT to DPT due to a very large amount of adipose tissue. Another patient underwent DPT from the beginning because the surgeon was unfamiliar with SPT. Two patients with thymic tumors adherent to the phrenic nerve and one patient with a suspected pericardial invasion underwent SRT. There was no switch to median sternotomy in any patient. The mean surgical duration was 194.9±47.7 min, and the mean intraoperative blood loss was 3.1±2.8 g. No intraoperative complications were encountered. One patient (6.6%) who underwent SPT exhibited transient paroxysmal atrial fibrillation postoperatively. Moreover, no postoperative crises or death was seen.
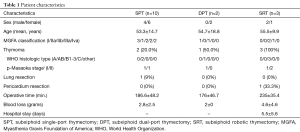
Full table
Discussion
MG is an autoimmune disease caused by the presence of antibodies to acetylcholine receptors and other receptors at neuromuscular junctions. Treatment options for MG include anticholinesterases, oral adrenocorticosteroids, immunosuppressants, adrenocorticosteroid pulse therapy, high-dose immunoglobulin therapy, blood purification therapy, and thymectomy.
Thymectomy was first performed in 1911 in Zurich by Ferdinand Sauerbruch on a 21-year-old MG patient who showed improvement in symptoms the following year, as reported by Shumacher and Roth et al. In 1944, Blalock et al. reported improvement in 20 MG patients following thymectomy (7). In 2016, Wolfe et al. reported the efficacy of thymectomy for MG in a multicenter randomized trial (8). Excision of the thymus with many germinal center-like structures has been considered to alleviate the symptoms of MG. In 1982, Masaoka et al. reported that extended thymectomy, or resection of the thymus with its surrounding adipose tissue, was more effective than simple thymectomy (9). Specifically, extended thymectomy for MG includes the resection of both superior poles of the thymus below the thyroid attachment site at the cranial end and the portion of the thymus in front of the bilateral phrenic nerves at the dorsal side, as well as all surrounding adipose tissues. This technique is currently employed at many facilities. Some reports even recommended maximal thymectomy, in which the periphytic adipose tissue is more extensively resected (10). In any case, additional resection of the surrounding adipose tissue instead of the thymus alone is considered vital for enhancing the efficacy of thymectomy in MG. In recent years, surgeons have come to use less invasive endoscopic surgical techniques than conventional median sternotomy to perform these procedures. Endoscopic thymectomy includes the transcervical approach via the neck region, video-assisted thoracoscopic surgery via the lateral intercostal space (lateral thoracic intercostal approach), and the subxiphoid approach.
Transcervical thymectomy was reported by Cooper et al. in 1988 (11). This method is an excellent, minimally invasive surgical technique that causes minimal pain; however, its use has not been widespread because of poor operative field of view and operability. Furthermore, the neck incision may not be cosmetically acceptable because it cannot be hidden by clothing. The lateral thoracic intercostal approach was first reported by Sugarbaker et al. in 1993 (12) and is currently the most commonly employed technique at facilities that offer endoscopic thymectomy, including robot-assisted surgery (13,14). With this approach, it is difficult to secure the operative field in the neck region and to completely resect the adipose tissue surrounding the superior lobes of the thymus. Furthermore, in the unilateral thoracic approach, a difficulty in verifying the location of the contralateral phrenic nerve complicates expanded thymectomy. A major drawback of this approach is that the intercostal space is traversed, which guarantees intercostal nerve damage from the concurrently inserted port. Moreover, approximately 10% of intercostal nerve damage cases will develop post-thoracotomy pain syndrome with lifelong pain and numbness (15). Bilateral chest pain and numbness may also occur when the bilateral lateral thoracic intercostal approach is used to perform extended thymectomy.
Thymectomy via the subxiphoid approach began with Kido et al. in 1999 (16). In 2012, the author and his colleagues reported SPT involving the use of single-port surgical instruments and CO2 insufflation into the mediastinum (1). An advantage of this approach is that it does not cause intercostal nerve damage because it does not traverse the intercostal space. In addition, with this approach, it is easy to verify the location of the bilateral phrenic nerves and to secure the operative field in the neck region within the field of view of the camera that is inserted in the midline of the body. On the other hand, the drawbacks include difficulty in verifying the location of adipose tissue on the pericardium in case of cardiomegaly, the need for familiarity with manipulations unique to single-port surgery, and the difficulty in suturing. The author opts for DPT or SRT with an additional intercostal approach in cases that require suturing. Although these methods cause intercostal nerve damage, the field of view from the subxiphoid region is better than that with the lateral thoracic approach in securing the operative field in the neck region and verifying the location of the bilateral phrenic nerves.
DPT results in less interference between surgical instruments, which allows good operability and easy suturing including pericardial patch closure (3). In Japan, DPT is commonly performed in more facilities than SPT. Facilities that are only in the beginning stages of offering subxiphoid thymectomies are recommended to start with DPT.
Recently, robot-assisted thymectomy has been reported to yield good results for MG (17,18); all these reports described a lateral thoracic intercostal approach, which is the usual case in the majority. Moreover, the existence of robotic joints that move in the same way as human wrist joints has facilitated surgical manipulations in the narrow mediastinum. In 2015, we reported robot-assisted thymectomy via the subxiphoid approach (4,19). To maximize the robotic performance, the target—in this case, the thymus—should lie between the left and right arms of the robot. In the lateral thoracic approach, the superior poles of the thymus do not lie between the left and right robotic arms. In contrast, with the subxiphoid approach, the entire thymus lies between the left and right arms; this maximizes the potential of the robot and allows easier surgical manipulation. SRT, which has good operability and operative field of view, could become the standard approach for robot-assisted thymectomy in the future.
Thymectomy via the subxiphoid approach for MG is an excellent technique for both surgeons and patients because the operative field in the neck region is secured, bilateral phrenic nerve identification is possible, cosmetic outcomes are superior, and pain is minimal. Further studies on the long-term therapeutic outcomes of subxiphoid thymectomy for MG are required.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Minimally invasive VATS thymectomy for Myasthenia Gravis” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.03.01). The series “Minimally invasive VATS thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Suda T. Subxiphoid single-port thymectomy. Asvide 2017;4:111. Available online: http://www.asvide.com/articles/1419
- Suda T. Subxiphoid robotic thymectomy. Asvide 2017;4:112. Available online: http://www.asvide.com/articles/1420
- Blalock A, McGehee HA, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland. JAMA 1941;117:1529-33. [Crossref]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Masaoka A, Monden Y. Comparison of the results of transsternal simple, transcervical simple, and extended thymectomy. Ann N Y Acad Sci 1981;377:755-65. [Crossref] [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. "Maximal" thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Detterbeck FC, Kim AW, Zielinski M. Looking in from above and up from below: new vistas in thoracic surgery. Innovations (Phila) 2012;7:161-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
Cite this article as: Suda T. Subxiphoid VATS thymectomy for myasthenia gravis. Video-assist Thorac Surg 2017;2:15.