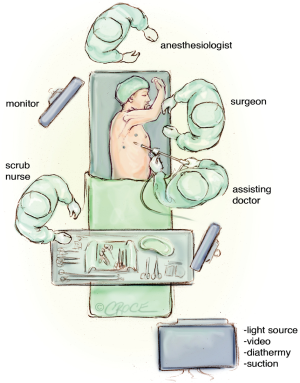
VATS, robotic lobectomy and microlobectomy—the future is just ahead?
Introduction
Jacobeus first pioneered the use of a thoracoscopy in 1910 for lysis of adhesions and drainage. Following that, in 1921, he published his extensive experience in pleuroscopy for diagnostic purposes (1). Over the next 60 years, minimally invasive procedures gained a foothold and the phenomenal success of laparoscopic surgery fuelled the interest in other specialties.
Video assisted thoracoscopic surgery (VATS) lobectomies have been instrumental in the evolution of thoracic surgical oncology since its introduction in the early 90s. Although there is no robust data to confirm or refute its superiority over open conventional lobectomy, there have been a number of meta-analyses which have shown that VATS is safe and feasible for those undergoing radical resection for cancer. Multiple observational studies have shown that the rates of post-operative morbidity are lower than for conventional surgery, in particular complications such as pain, incidence of pneumonia and cardiac arrhythmias; VATS patients also have reduced length of stay compared to open procedures. Furthermore, more patients undergoing VATS receive adjuvant treatment and at higher doses (2). The SCTS Thoracic surgery database for the UK and Eire have shown mortality rates of 1% for VATS compared to 2% for conventional open procedures (3).
VATS lobectomy
The definition of VATS in the early studies varied according to the surgeon or centre performing them. The consensus definition, first described in the CALGB 39802 trial (3), of a VATS lobectomy is as follows (4):
- One 4- to 8-cm utility access port;
- Between one to three 0.5-cm port incisions;
- Used videoscopic guidance;
- Traditional hilar dissection;
- No rib-spreading.
Concerns were raised about the oncological adequacy of VATS. This is because of the perceived idea that lymphadenectomy in VATS would be more difficult. Cao et al. (5) and Yan et al. (6) have shown that VATS is safe and the oncological outcomes are similar to conventional open lobectomy. Mediastinal lymph node dissection (MLND) during VATS lobectomy has shown to be equally efficacious to open lobectomy (7).
However, the demand for VATS procedures is patient-driven. The arguments in favor of VATS lobectomy include cosmesis, less postoperative pain, shorter length of stay, and relative lower overall cost (8). Despite the apparent benefits of the minimally invasive approach, the uptake for VATS amongst surgeons is still low. Data published by the SCTS in the UK show that only 30% of lung resections are performed via VATS, although that varied from unit to unit. In the US, the figure is closer to 50%, while in Japan, >50% of cases are performed with VATS (9). This is multifactorial but can be broadly put down to the following reasons (10):
- Technical issues, 2D vision and limited maneuverability;
- Lack of adequate training;
- Concerns about major vascular injury and control of bleeding.
There is no doubt that the learning curve is initially steep but once surgeons were comfortable performing minimally invasive radical resection for lung cancer, the envelope was pushed further out. Smaller and smaller incisions were made and the number of ports decreased from the initial 3-4 port to 1-2 port techniques. The utility incisions were usually made in the intercostal spaces to allow multiple instruments to be used for dissection and retraction. However, movement of instruments in and out of the port sites may cause neuropraxia, which may give rise to long-term neuropathic pain that can be disabling. Additionally, removing a large, air-trapped lobe or a lobe containing a large tumour from the same intercostal space can compound the problem further.
Uniportal VATS lobectomy
Single-port pulmonary resections were initially described by Rocco and colleagues in 2004 and they have published their 10-years experience in uniportal VATS surgery for diagnostic and therapeutic procedures (11).
Uniportal VATS lobectomy has been pioneered by Gonzales-Rivas in Coruna and is now being used for complex resections including pneumonectomies, sleeve resections, redo surgery and tumours with chest wall involvement. The operative time was higher in patients with advanced tumours but duration of chest tube drainage, length of stay and complications were similar (12).
Subxiphoid utility incision—a step away from the intercostal spaces
In parallel there has been a renewed interest in subxiphoid surgery which is not a new concept in thoracic surgery. In 1999 a technique was described for metastasectomy by VATS which included a subxiphoid port to allow manual palpation of all lobes in both hemithorax without the need for a mini-thoracotomy (13,14). This subxiphoid approach also enabled mediastinal masses to be removed with a single incision (15).
The subxiphoid approach has more recently been expanded with novel subxiphoid uniportal approaches for thymectomies and lobectomies from innovators in the Far East (16-20). Most recently Jiang and colleagues from the Shanghai Pulmonary hospital published a series of 153 cases of lobectomies of every lobe and 48 segmentectomy using this approach (21).
This technique essentially obviates the need for making a large incision in the intercostal space and thus, reduces damage to the intercostal muscles and neurovascular bundle. For example, in order to remove tumours of 2–5 cm, it is often necessary to incise the intercostal muscles by 8–10 cm to allow the ribs to separate without causing fractures. This is more so in larger tumours and patients with air trapped lungs.
Additionally, patients tolerate incisions in the upper abdomen much more than in the intercostal space—this will allow them to perform their deep breathing exercises and cough and clear their secretions. Another way of minimising post-operative pain is to place the intercostal chest tube to be placed via the subxiphoid port. Thus, the incidence of long-term neuropathic pain should be much less.
Another benefit of the subxiphoid port is that either pleura can be entered easily under direct visualisation. This incision allows a 12 mm CO2 port to be placed and thus, can be useful for CO2 insufflation, retraction and stapling using the conventional endo staplers. Alternatively, A wound protector system can be used (Alexis Wound Retractor; Applied Medical, Rancho Santa Margarita, CA, USA). The port enables access to all the hilar structures with minimum articulation of the endo staplers and also allows the fissure to be developed when using staplers. Naturally, VATS using the subxiphoid port has evolved into a totally uniportal VATS without any intercostal incisions.
Robotic lobectomy
VATS techniques using conventional endoscopic instruments only allows two-dimensional (2-D) visualization although more recently, 3-D cameras and monitors along with 3-D glasses have been used. There may be a variety of reasons why surgeons are not keen to take up VATS lobectomy and they are mostly technical. The main drawback of VATS has been the 2-D vision with minimal range of amplification, which can make hilar and fissural dissection more difficult especially since depth perception is also limited. Hand-eye coordination can be difficult as the monitor is usually further away from where the surgeon is working.
Newer articulating instruments including endo staplers and cameras have helped to overcome some of the difficulties of having 2-D vision; and this allows dissection around the vessels and lymphadenectomy to be performed safely. These instruments however, have not really been able to completely replicate the 360-degree movement in the operators’ wrists, and the ergonomics still have a long way to go, especially within the limited confines of the thoracic cavity. Furthermore, pivoting the instrument in the intercostal spaces can cause significant neuropraxia, which hinders the patients’ recovery. Fine dissection in the mediastinum can be more difficult because tremor amplification. Another consideration is the larger radius of the movement curvature inside the chest when pivoting an instrument (22,23).
Advocates of robotic lobectomy state that this procedure addresses some of the concerns mentioned above. The superior imaging and 3-D camera offers unparalleled vision and magnification. The robotic endo-wrists allow precise movements of the instruments inside the patient, following the natural movements of the surgeon’s wrist. Advantages of robotic compared to conventional VATS include the additional four degrees of freedom (internal pitch, internal yaw, rotation and grip), the elimination of the fulcrum effect, reduced human tremor and improved ergonomic position for the surgeon (24).
Hand-eye coordination is maintained as the monitor and endo-wrists are located on the same console. The camera is manipulated at the console using the endo-wrists and a dedicated foot pedal. It allows variable magnification, high-definition stereoscopic images to the monitor, which may compensate for the absence of haptic feedback (25).
Although there is a paucity of robust randomized controlled trial data comparing robotic lobectomy to VATS or even thoracotomy—a few studies from the US and Europe report comparable perioperative outcomes to the results of a recent systematic review on conventional VATS (6).
Complications types and the rates are comparable to VATS lobectomies and perhaps lower than open procedures. There is no randomized controlled trial to assess the oncological outcomes following robotic lobectomy but Park et al. published a retrospective multi-institutional review on 325 patients undergoing robotic lobectomy for early-stage NSCLC. The conversion rate to thoracotomy was 8%, with an overall morbidity rate of 25.2%. In hospital death was only 0.3% and the median length of stay was 5 days. The overall 5-year survival was 80% after a mean follow up period of 27 months. The oncological effectiveness can only be ascertained when longer term data is available. However, the rate of upstaging stage I NSCLC is 21% (26), which is much higher than the 11.6% reported for VATS and 14.3% for open procedures (27).
The limitations of robotic lobectomy include the initial period where the learning curve is steep. However, a figure of 20 cases is quoted by three studies as the volume required to attain necessary skills in robotic surgery (5). Results from Cao’s systematic review identified that highest conversion rates and operating times were from institutions that performed <30 cases. Therefore, adequately trained specialised anaesthetists, scrub staff, and assistants are mandatory to enable a robotic lobectomy program to achieve a satisfactory outcome. Furthermore, these cases should be performed in high-volume tertiary care centres to allow effective multi-specialty usage of the robot. This subsequently increases efficiency and savings especially in terms of cost for initial capital, consumables and training. If two consoles are available—training, teaching and proctoring in robotic lobectomy is possible. In the UK, the first two centres to start a robotic lobectomy program are in the North East of England (Freeman and James Cook University Hospitals) and the regular teaching/training of registrars/residents will now be the next phase. We should look to our urology colleagues in the UK where robotic surgery has been incorporated into the curriculum for its residents and trainees.
In a nationalized public healthcare system such as the National Health Service, one of the primary considerations of a clinical commissioning group which funds hospital trusts would be the cost effectiveness of a procedure. The initial outlay or capital cost would be the biggest—Park et al. reported that the initial capital cost of the da Vinci robot system was about a million USD in 2008. The costs for each operation are USD 3—4,500 more when compared to VATS (28). However, thoracotomy costs are higher as the patients have longer intensive care and in-hospital total length of stay (29). Indeed, NHS England are currently reviewing the cost-effectiveness of robotic lobectomy and this potentially may have an adverse impact into the future provision of services in the UK.
In summary, robotic lobectomy is feasible and can be performed safely for selected patients in selected high volume tertiary care centres. However, high costs and the paucity of robust evidence in terms of its superiority over VATS for peri-operative outcomes and long-term oncological adequacy is limiting its utility especially in public health care systems.
Microlobectomy—smaller incision than VATS or robotic surgery
Microlobectomy is one of a range of novel techniques currently under evaluation—created by a group of VATS lobectomists internationally and has some advantages for experienced VATS surgeons.
Firstly the technique of the lobectomy is not too dissimilar to the more usual VATS lobectomy. Our group has used this technique to perform resections of every lobe (both anterior and posterior approach) and we recommend that surgeons interested in trying this technique place their 5 mm ports in the usual positions. We have also performed segmentectomy and sleeve resections safely (Figure 1: right upper lobe sleeve microlobectomy) and a right pneumonectomy where a subxiphoid extraction was, in our view, particularly advantageous.

Our group uses CO2 insufflation, which allows more space in the hemithorax and aids with lung collapse at the start of surgery especially in patients with air-trapping. Furthermore, the dissection and safe placement of a subxiphoid port is facilitated. Depending on surgeon preference, if, after the initial steps of the operation it becomes less useful, the CO2 could be turned off. Of note our technique is a fully endoscopic technique and therefore forceful or uncontrolled suction may cause lung inflation. We prefer intermittent suction or the use of rolled-up tonsil-swabs to remove small amounts of blood intraoperatively.
Operative technique
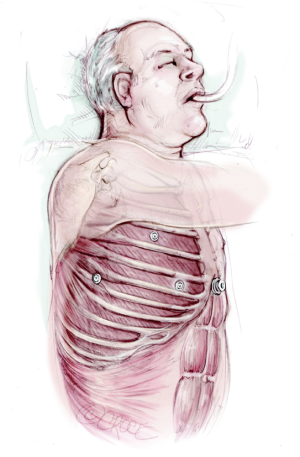
The patient is intubated with single lung isolation and positioned in a standard lateral position (Figures 2,3). The patient should be positioned in the same position that the operating surgeon is familiar with, for their usual VATS technique. The only modification is that the xiphisternum, costal margins and the midline down to the umbilicus is marked prior to positioning. After turning into the lateral position, good access to the subxiphoid area must also be ensured (Figure 4).
For patients undergoing an anterior approach lobectomy, the first port is placed in the 4th intercostal space between the inferior angle of the scapula and the nipple. In a normal VATS lobectomy this would be the area of the utility incision and in uniportal surgery this is the location of the single incision. For microlobectomy a 5 mm port is inserted here. Chest entry is gained under vision with the Kii-Fios first-entry port (Applied Medical, California, USA) with CO2 running at 5 litres per minute (Figure 5). The camera is placed in the centre of the clear plastic trocar and the port is inserted under vision. As soon as the trocar breaches the pleura the CO2 pushes the lung away and this can be seen endoscopically. If there are adhesions, these will be seen and the CO2 will facilitate their separation from the chest wall.
Once the chest has been entered, the hemithorax is insufflated to a pressure of 5–10 mmHg. High CO2 levels may cause hypercarbia, high airway pressures or hypotension so the flow rates may have to be adjusted temporarily to allow these parameters to stabilise.
The camera is then directed down to look at the inferior border of the sternum and the antero-medial diaphragm. A 20 mm skin incision is made vertically just below the xiphisternum, then under vision the soft tissue is dissected down to the tip of the xiphisternum which marks the cranial portion of the linea alba. This is incised vertically for 15 mm. It is important not to deviate into the rectus abdominis muscle as this will cause unnecessary post-operative pain. A finger is then passed cranially directly posterior to the xiphisternum and up behind the sternum as far as possible. This is similar to the move a surgeon makes prior to performing a sternotomy. The finger is then moved laterally into the hemithorax under direct vision.
Once the pleura is breached this can be followed with a 12 mm port. The diaphragm is always well below this entry point due to the CO2, and we have not encountered any subdiaphragmatic entries with this method.
After the subxiphoid port has been placed, two further 5 mm ports are made according to the usual positioning of the surgeon’s further ports. Often this corresponds to the ports described as the standardized anterior approach by Hansen and Peterson (18,19), but the operation has also been performed safely using the posterior approach (20), with the camera port first being placed posterior to the inferior border of the scapula.
The operation is then conducted in the usual fashion using 5mm instruments. Retraction can be achieved through the subxiphoid port, and stapling can either be achieved using the 5mm Dextera Microcutter for vascular structures (Dextera Inc, Redwood City, CA, USA), an energy device, or if none of these are available, a 12 mm standard stapling device can be used from the subxiphoid port. This port is conveniently located at the anterior end of the oblique fissure on both sides and thus enables good access to the hilar structures for stapling. Further information on the surgical technique and useful instruments can be found at www.microlobectomy.com.
At the end of the procedure an endo bag is placed from the subxiphoid port and then once the specimen is in the bag, under vision, the linea alba is extended as far as necessary to remove the tumour. The chest tube is inserted through the subxiphoid port and this wound is then closed, taking care to suture the linea alba under vision throughout its length to prevent an incisional hernia.
There is a wide range of novel instrumentation which facilitates minimally invasive surgery. The Covidien Single Incision Laparoscopic Surgery (SILS)® dissector is a 5 mm instrument that can articulate to 80 degrees. This is particularly useful for dissecting around vessels. The Dextera Micro Cutter® is a stapling device that has recently received FDA approval. It is licensed for the transection of vessels up to 2 mm in clamped wall thickness and is particularly useful for small segmental vessels. In addition to its narrow diameter it is also able to articulate to 80 degrees. There is now a wide range of high quality 5 mm cameras with a resolution not dissimilar to 10 mm cameras. While 3D imaging is not yet possible in 5 mm we believe that these 5 mm cameras are very versatile and suitable for anatomical lung resection. Additionally, for the sleeve resections, 5 mm endoscopic needle holders can be used (the sutures can be inserted via the subxiphoid 12 mm port).
In VATS lobectomy, safety is paramount and emergencies should be planned for. A key step in addressing significant bleeding in endoscopic lobectomy is the ability to apply pressure to the area of bleeding with a wide based swab or sponge stick. We routinely use one or two rolled tonsil swabs in the chest. Microlobectomy does not allow for the rapid insertion of a sponge stick, but we find that it is possible to grasp the tonsil swab in the chest and then apply pressure to the area of concern. An alternative method is to grasp the lung and place this over the area of bleeding. If bleeding is controlled then conversion to thoracotomy can easily be performed. We have also easily converted to the standard VATS approach in bleeding simply by extending the size of the ports and creating a utility incision, and have been then able to deal with bleeding by VATS and complete the operation endoscopically.
Adhesions are not a contraindication to microlobectomy. The CO2 allows the separation of all but the most dense of adhesions and allows entry into the chest. As the first port has the camera in the trocar, if adhesions are seen, then a sweeping action of this port under vision is a very safe way to create some space in the chest prior to the insertion of further ports. We have yet to convert to VATS to complete the case due to adhesions.
As the operation utilizes the same view as a surgeon’s usual approach, we have found that lymphadenectomy is no different to a standard VATS lobectomy. The nodes may be removed through the subxiphoid port and may be removed in a bag if they are large. The subxiphoid port is also useful for retraction for station 7. A small bag may be inserted into the chest, and retraction performed until the end of the lymphadenectomy and then the bag removed at the end of this part of the operation.
All operations have weaknesses and microlobectomy is no exception. Using the subxiphoid port for retraction rather than 2nd or 3rd instruments through the utility incision is sometimes cumbersome and some practice and experimentation with 5 mm retraction devices is required. Suboptimal retraction can lead to delays in the operation. The closed chest technique does require valved suction and brief bursts of suction, as more prolonged periods of suction does cause lung re-inflation.
So far, 72 patients have undergone microlobectomy in 6 hospitals sited in the UK, US and Denmark. A total of 40/72 of cases (55.5%) involved the upper lobes. The median operating time is 180 mins (range, 94–285 mins) and blood loss was 118 mL (range, 5–800 mL). There was a 4.1% conversion rate for bleeding and 2.8% conversion to VATS rate (by extending a port to become a traditional utility incision). The median hospital stay was 3 days (22% of patients going home on post-operative day 1). The other common complications were pneumonia (14%), prolonged air leak (7%), atrial fibrillation (4%) and prolonged intubation (4%).
Our most important weakness is that we present no evidence that microlobectomy is superior to any other endoscopic lobectomy technique or indeed to a thoracotomy. We believe that at this stage it is for individual surgeons to select their own techniques from the range available. We present this article and additional learning resources to enable surgeons to try this method as part of their own journey to find their own optimal technique. This weakness is not new and there is no compelling evidence of superiority of any other one endoscopic lobectomy technique over another currently. Indeed such is the doubt over the superiority of endoscopic lobectomy versus lobectomy by thoracotomy that there are currently several randomized controlled trials recruiting internationally including a large multi-centre randomized trial called VIOLET aiming to recruit 495 patients in the UK to answer this question (31).
Conclusions
Minimally invasive surgery has revolutionised the way we treat primary lung cancer. There are a variety of different techniques, approaches, instruments and modalities that are constantly evolving to enable safer and easier surgery; as well as to improve the patient experience not just in the immediate post-operative phase in terms of length of stay, pain and complications but also for the longer term so that adjuvant therapy can be administered as soon as possible after surgery. While the uptake of VATS or robotic surgery in the UK and EU is low, there is still some room for growth. There is a paucity of randomised control trial data to compare VATS with robotic and/or open procedures but hopefully the upcoming VIOLET study will be able to address some of these key questions. However, we know from observational data and small RCTs, VATS and robotic lobectomy is safe, feasible and reproducible.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luca Bertolaccini and Piergiorgio Solli) for the series “VATS: the age of maturity” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.01.02). The series “VATS: the age of maturity” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yoneda KY, Mathur PN, Gasparini S. The evolving role of interventional pulmonary in the interdisciplinary approach to the staging and management of lung cancer. Part III: diagnosis and management of malignant pleural effusions. Clin Lung Cancer 2007;8:535-47. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- The Society for Cardiothoracic Surgery in Great Britain & Ireland. Second National Thoracic Surgery Activity & Outcomes Report. 2011. Available online: http://scts.org/wp-content/uploads/2016/10/Thoracic_BlueBook_2011.pdf
- Swanson SJ, Herndon JE, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg 2008;85:S710-5. [Crossref] [PubMed]
- Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2008: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2010;58:356-83. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Paci M, et al. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg 2001;136:783-8. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Liu CY, Lin CS, Liu CC. Subxiphoid single-incision thoracoscopic surgery for bilateral primary spontaneous pneumothorax. Wideochir Inne Tech Maloinwazyjne 2015;10:125-8. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6.
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Mohamed Mydin MI, El-Saegh MM, Nardini M, et al. VATS, robotic lobectomy and microlobectomy—the future is just ahead? Asvide 2017;4:110. Available online: http://www.asvide.com/articles/1418
- Lim E, Brush T, Rogers C, et al. 189 VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer, a multi-centre randomised controlled trial with an internal pilot: the VIOLET study. Lung Cancer 2016;91:S68-9. [Crossref]
Cite this article as: Mohamed Mydin MI, El-Saegh MM, Nardini M, Dunning J. VATS, robotic lobectomy and microlobectomy—the future is just ahead? Video-assist Thorac Surg 2017;2:14.