Robotic thymectomy: technical tips
Introduction
Thymectomy constitutes a widely accepted therapeutic option in the multidisciplinary management of MG and the cornerstone in the treatment of thymic tumors (1-3).
A variety of surgical approaches for thymectomy has been described, ranging from open to minimally invasive ones.
The introduction of robotic-assisted surgical systems has brought clear technical advantages over standard video-assisted thoracoscopy, particularly for surgical application in remote to reach or narrow anatomical regions, such as the mediastinum (4).
Patient selection and workup
Main indications for robotic thymectomy are patients with MG and patients with early stage thymic tumors associated or not with MG.
During pre-operative evaluation it is important to investigate if there are symptoms or clinical signs that could be related to MG and to evaluate the serum titer of antibodies against acetylcholine receptor. If negative, antibodies against the muscle specific receptor tyrosine kinase (anti-MuSK Ab) should be tested; there is evidence that thymectomy can be less effective in MG-patients with positive serum titer of anti-MuSK Ab (5).
There isn’t a gold standard in surgery timing but it seems that in case of recent onset of symptoms there is higher possibility of remission or improvement after thymectomy (6). Age over 50 years or antibody-negative disease are relative contraindication to thymectomy in non thymomatous MG (7,8).
All patients should be evaluated with a contrast-enhanced CT scan of the chest. Magnetic resonance and/or PET-CT scan can also be performed. Extensive adhesions related to prior pleuritis or thoracic surgical procedure may preclude a robotic approach.
Functional assessment should be completed with pulmonary function tests, complete blood examination and electrocardiogram.
Pre-operative preparation
Neurological assessment prior to surgery should be always performed to evaluate presence of active or significant symptoms of MG or to optimize the medical treatment.
The patient is under general anesthesia intubated with a double-lumen endotracheal tube, for selective single-lung ventilation during the operation, and is monitored by electrocardiogram, arterial line, pulse oximeter and urine output.
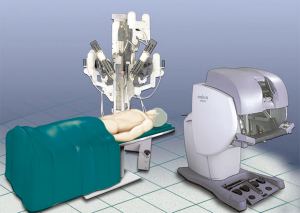
The patient is positioned left- or right-side up (depending from the side of operation) at a 30-degree angle with a bean bag. One arm is positioned along the body and the other is on a support parallel to the bed to better expose the axillary region on the side of operation (Figure 1). The robotic cart is positioned on the right side of the bed (left side for right-side approach) with a 45° angle (Figure 2).
The operative field should always be draped for an eventual open conversion.
Equipment preferences and cards
Different robotic systems have been developed in the past years but the most widespread is the Da Vinci robotic system (Intuitive Surgical, Inc., Sunnyvale, Calif).
It consists of a surgeon’s computerized console, a vision system and a patient-side cart that supports the robotic arms (Figure 3). The surgeon controls the system sitting at the console far from the patient. The console represents the interface between surgeon and robotic system. The surgeon sees the operative field through a binocular localized in the upper part of the console and his fingers grasp the master controls below the display realizing the movements of robotic instruments. The system translates the movements of hands and fingers into precise, identical, and real-time movements of surgical instruments inside the patient. A support makes the movements comfortable and is furnished with several buttons for the regulation of various functions like the type of vision (2-D or 3-D view) the type of optic (0°–30°). Moreover, the system is equipped with a tremor filtering that allows for extremely precise movements. At the bottom of the console a series of 5 pedals permits other controls such as the activation of electrocautery, the variation of focal point of the camera, etc. (Figure 4).
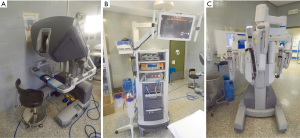
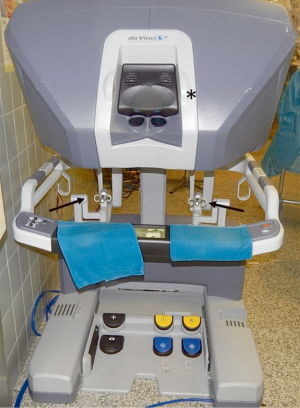
The vision system contains the video components: a monitor that allows the operating-room personnel to view the intervention, and two boxes for control of the video-camera and for the balancing of luminosity and contrast of the image. A system for the supply of CO2, and its intracavitary pressurization can be placed in this tower.
The patient-side cart supports the arms of the robot, the central one holding the 12-mm diameter optic. The left arm has an EndoWrist instrument that grasps the thymus; the right arm has an Endo-dissector device with electric cautery function (or a Harmonic ultrasound dissector) used to perform the dissection. The surgical instruments are articulated with the main arm and they are designed with seven degrees of motion and a 360° rotation, which mimics the dexterity of the human hands and wrist.
Procedure
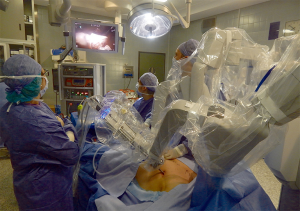
A 12-mm camera port for the three-dimensional stereo endoscope is introduced through an incision on the fifth intercostal space on the anterior/midaxillary line and two additional 8 mm thoracic ports are inserted; one on the third intercostal space on the midaxillary region and another on the fifth intercostal space on the parasternal space. After placing the first port, it is advantageous to use the camera to help placing the other two ports so that their trajectory line is correct and to avoid lesion of the heart or pericardium when placing the parasternal port. Two arms of the da Vinci system are then attached to the two access points and another arm is attached to the port-inserted endoscope (Figure 5).
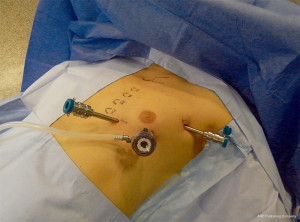
The hemithorax is inflated through the camera port with CO2 (pressure ranging between 6 and 10 mmHg) to obtain a clear view within the chest and to allow an easier dissection as it extends the mediastinal space.
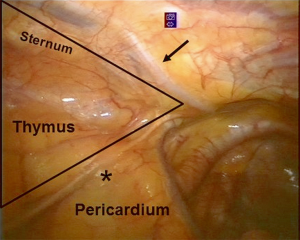
First, a careful exploration of the mediastinal left pleural space is performed to exclude the presence of any pleural implants in case of thymic tumors. Then, the field of the surgical dissection is individuated in a triangle area with the basis at the bottom of pericardium where the fat tissue of the left and right pericardiophrenic angles is localized. The lateral borders of the triangle are delimited by the mammary vessels anteriorly and by the left phrenic nerve posteriorly, with the apex located in the neck (Figure 6).
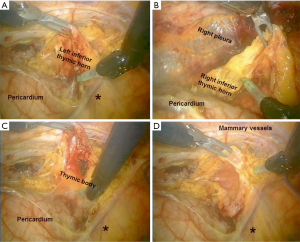
When a left-sided approach is performed, the dissection starts inferiorly at the left pericardiophrenic angle and continues along the anterior border of the phrenic nerve. All anterior mediastinal tissue, including fat, is isolated from the phrenic nerve. Caution must be paid not to damage the nerve, therefore during dissection it may be useful if the table-site assistant puts a hand on the patient abdomen to perceive any hemidiaphragm contraction. The left inferior horn of the thymus is then located and dissected from the pericardium. Subsequently, the thymic gland is separated from the retrosternal area until the right mediastinal pleura and the right inferior horn are found (Figure 7A-C). The lower part of the thymus is then mobilized upwards until the level of aortic arch. At the top of the mediastinum, the pleura is incised in the area delimited by the mammary vessels in the anterior limit and by the phrenic nerve in the posterior limit (Figure 7D). The dissection continues upward to the neck until the superior horns are identified and divided from the inferior portion of the thyroid gland by a blunt dissection (Figure 8A). Grasping and pulling the superior horns below, the innominate vein is then identified and dissected along its border up to the point where the thymic veins are identified, clipped and divided (Figure 8B).

The thymus gland, the anterior mediastinal, and the neck fatty tissues are radically resected and the specimen is placed in an Endobag and removed through trocar incision.
In the right side approach, dissection begins from the cardiophrenic angle and the mediastinal pleura is incised just anterior and medial to the right phrenic nerve, then continues upwards till all anterior mediastinal tissue is separated from the nerve. At this point starts the dissection onto the sternum and the division of the pleura along the right internal mammary artery and veins from the origin all the way to the diaphragm. The next step involves elevating the thymus off the pericardium, starting from the inferior horns and heading upwards until the left brachiocephalic vein is encountered. The thymus is dissected off the anterior aspect of the vein and the thymic veins are identified, clipped and dissected. Then the superior horns are identified and divided from the thyroid gland. The left pleura is then opened and after the left phrenic nerve is identified, the dissection of the thymus is completed.
After the hemostasis, a 28F drainage tube is inserted through the port of the fifth intercostal space, the lung is inflated, and the other wounds are closed.
Role of team members
Surgical team should be composed by 2 surgeons, 1 anesthetist, 1 scrub nurse and 1 operating room nurse. One surgeon is at the robotic console, controlling the robot. The other is table-site surgeon that must perform the incisions and introduce the ports. Moreover, he must be able to perform the connection between the robot and the ports and to rapidly undock the system and perform an emergency sternotomy in case of uncontrollable vascular bleeding or any other complication. The scrub nurse must be trained in the use of robotic material and able to exchange the robotic instruments. The anesthesiologist should be experienced in the management of patients undergoing thoracic procedures and of patient with MG (Figure 9).
Post-operative management
The patient is generally extubated in the operating room and, after an adequate period of observation, returns to the ward. In some cases (i.e., patients with MG not well controlled pre-operatively) the patient may be transferred to the ICU for monitoring.
The chest drain is removed if the postoperative chest X-ray shows normal findings and the amount of pleural fluid is permissive, generally 24 hours after operation. If neurological evaluation is satisfactory, the patient is discharged 48–72 hours after surgery.
Tips, tricks and pitfalls
Different issues regarding robotic thymectomy are still matter of debate.
First is the better side to perform thymectomy. Authors supporting the left-sided approach point out that the left lobe of the thymus gland is usually larger and extends down to the pericardiophrenic area, and that the aortopulmonary window and the region below the left innominate vein are frequent sites of ectopic thymic tissue. The thymus may also extend lateral to or under the left phrenic nerve, or descend totally or partially posterior to the innominate vein (9). Moreover, the right phrenic nerve is protected by the superior vena cava in the high mediastinum and may be identified and easily followed in the lower part (10,11).
Authors who prefer a right-sided approach emphasize the larger operative field, the better visualization of the venous confluence by following the superior vena cava, the easier visualization of the aortocaval groove and a better ergonomic position to accomplish dissection making it easier in the early part of the learning curve (9,12,13).
Anyway, the approach should be tailored on the patient’s anatomy in order to perform a complete dissection of all the thymic tissue and the mediastinal fat which may contain foci of ectopic thymus in patients with MG (14). A particular care should be used when left innominate vein is dissected in order to avoid a major bleeding: when a small thymic vein is encountered and clipped, it is mandatory to search for a second vein that usually is present at the level of left innominate/superior vena cava angle. In about 5% to 10% of cases an anatomical variation is present: the most common is the upper left horn running behind the innominate vein and over the subclavian or carotid artery. In case of right sided approach, the dissection of the abnormally positioned left upper horn may be very difficult.
Correct selection of patients is mandatory in case of thymomas, in order to avoid complications and achieve the best result from the oncological point of view.
Cheng et al. proposed some radiological criteria for candidates to minimally invasive thymoma resection: the location in the anterior mediastinum, the tumor encapsulation, a distinct fat plane between the tumor and vital organs, the existence of residual normal appearing thymic tissue, no mass compression effect and unilateral tumor predominance, particularly for tumors of dimension greater than 3 cm (15).
A large tumor size (3–5 cm) may not be an absolute contraindication, however, it could make manipulation more difficult, with increased chance of open conversion or prolonged operative time (11).
Thymomas should be resected using a “no-touch” technique, removing the tumor en-bloc with the surrounding thymus and fatty tissue and care should be taken to avoid rupture of the capsule with increased risk of pleural dissemination (16). This technique requires a more complex and accurate dissection, thus a longer learning curve and operative time (11).
If an initial attempt of minimally invasive approach is deemed by the surgeon to be unlikely to be completed, both for the risks of the procedure itself or possible violation of any principle of oncologic safety, open conversion is mandatory.
Major disadvantages have been described regarding robotic surgery. Some authors pointed out the lack of tactile feedback that could theoretically increase the risk of damaging delicate structures. Anyway, this disadvantage seems to be widely compensated by the superior view of the operating field through the 3-dimensional vision (11,17). Another disadvantage concerns the placement of the surgeon, away from the patient and operating at a non-sterile console (17). Thus, another surgeon able to perform an emergency conversion needs to stay sterile near the robot.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Minimally invasive VATS thymectomy for Myasthenia Gravis” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.02.01). The series “Minimally invasive VATS thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthesia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Sussman J, Farrugia ME, Maddison P, et al. Myasthenia gravis: Association of British Neurologists' management guidelines. Pract Neurol 2015;15:199-206. [Crossref] [PubMed]
- Alkhawajah NM, Oger J. Treatment of Myasthenia Gravis in the Aged. Drugs Aging 2015;32:689-97. [Crossref] [PubMed]
- Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Deen S, Farivar AS, Louie BE. Thoracic techniques: robotic thymectomy for thymoma. Indian J Surg Oncol 2013;4:132-7. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
- Zieliński M, Kuzdzal J, Szlubowski A, et al. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann Thorac Surg 2004;78:253-8. [Crossref] [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
Cite this article as: Marulli G, Comacchio GM, Rea F. Robotic thymectomy: technical tips. Video-assist Thorac Surg 2017;2:6.